Early breast cancer irradiation therapy options
By Cynthia Biron Leiseca, RDH, EMT, MA
Most patients diagnosed with early breast cancer (Stages 0, I, II) are told that they will undergo radiation (called "irradiation therapy") in addition to lumpectomy. This combined treatment is known as breast-conserving therapy (BCT). For early breast cancer, several irradiation therapy treatment modalities may or may not be combined with chemotherapy.
This article provides an overview of the types of irradiation therapies currently available or in clinical trials, and also the latest research on proton irradiation therapy.
The first thought many women have when diagnosed with early breast cancer is: "I just want to have a bilateral mastectomy without radiation and never have to worry about it again." While that may be the best treatment for some women, it is not the best treatment for most women.1
--------------------------------------------------------------------
Consider reading these articles
- IORT: A treatment option for early stage breast cancer
- Sisterhood: When diagnosed with breast cancer, RDH finds sisters among patients
- Seeing red
--------------------------------------------------------------------
A study conducted in California included112,154 women with Stage I or II breast cancer who were treated with either breast-conserving therapy (BCT), which consisted of a lumpectomy and irradiation therapy, compared with those who had mastectomy alone. The women who had BCT had improved overall survival from any type of death, as well as survival specifically from breast cancer compared to the women who had bilateral mastectomy alone.1
The breast cancer-specific survival was most beneficial to women who were 50 or older and had hormone-positive-receptor breast cancer (hazard ratio = 0.86, 95% CI = 0.82-0.91) compared to women who were under age 50 with hormone-negative-receptor breast cancer (hazard ratio = 0.88, 95% CI = 0.79-0.98). This trend appeared among all the subgroups in the study and demonstrates that BCT with irradiation therapy remains an effective alternative to mastectomy for early-stage breast cancer in women of all ages and hormone-receptor types.1
In a survey of women ages 26-40 who had bilateral mastectomies, all stated their main reason for choosing bilateral mastectomy when only one breast was affected was to extend their lives. All of the patients knew that research has shown that most women with early breast cancer will die of something else. They also knew that there was only a 2% to 4% chance they would get a new cancer in their other cancer-free breast (if they did not have the BRCA1 or BRCA2 gene) and that removing the second breast would not increase their life span. Removing the second unaffected breast does not address the greatest concern: metastasis of the original cancer in the first breast affected with cancer.2
When do doctors recommend mastectomy?
Women are usually given all of the treatment options, and they must make the choice of which treatment to have. The benefits and the possibility of any adverse effects associated with each of the treatments are presented to the patient. Perhaps the most stressful part of the whole process of dealing with a diagnosis of early breast cancer is deciding on the type of treatment, since there is always some uncertainty on how well a patient will tolerate a particular treatment.3
Doctors often recommend a bilateral mastectomy to women who have tested positive for BRCA1 and/or BRCA2 genes since such patients have up to a 57% risk of getting an aggressive form of breast cancer. Bilateral mastectomy reduces the risk of breast cancer by 90%. The combination of bilateral mastectomy and removal of both ovaries (complete hysterectomy) reduces the risk of breast cancer by 95%.4
Mastectomy is also recommended when a breast has multiple tumors. It is also recommended if a breast irradiated for a previous cancer presents with a recurrence. But when the original cancer treatment was lumpectomy and partial breast irradiation, re-irradiation treatment is an option.5
- Whole breast irradiation -- Some women with early breast cancer choose mastectomy to avoid radiation therapy that must be part of BCT and whole breast irradiation (WBI). WBI external beam radiation for five days a week for five to seven weeks has been the standard of care for many years. The type of radiation that has been used in the standard of care is photon radiation. Those having more than five weeks of radiation usually receive tumor bed boost irradiation on the sixth or seventh week.
The length of treatment time may present a real inconvenience to patients, and the adverse effects of fatigue as well as other side effects can be debilitating. Long-term associated risks of radiation to healthy tissue and organs in the region of the tumor (small but real risks) may dissuade some patients from choosing breast irradiation treatment.6
- Left breast cancer and heart disease -- Women receiving irradiation therapy for left breast cancer are at greatest risk for heart disease from WBI. The lifetime risk is 0.05% to 3.5% and is dependent on how the radiation was delivered and if the women already had or will have underlying risks for heart disease.
The studies conducted were on patients receiving irradiation therapy 20 years ago. There are improved methods today of controlling photon irradiation from reaching the lungs and heart, but it is impossible to completely avoid photon radiation from reaching the heart in left breast treatment.7
Types of radiation and the latest research
Although WBI therapy of five to seven weeks is the standard of care, a shorter, three-week course of treatment has been studied and could prove to replace the current five to seven week standard course of treatment.
In the shorter course of treatment, photon radiation is given in larger doses and is called hypofractionated radiation. It causes less radiation to surrounding healthy tissue, according to the 10-year trials conducted with 4,451 women treated at 35 centers in the United Kingdom. Two trials occurred simultaneously, START A was the standard treatment and radiation dosage over a five-week course; START B was the hypofractionated dosage over three weeks. After a 10-year follow-up, cancer recurrence was the same in both groups. But the group in START B had significantly less damage to surrounding healthy tissue outside the region of the tumor bed. The three-week, shorter course is more convenient and less expensive.8
- External partial breast irradiation therapy -- In early breast cancer, up to 90% of recurrent breast cancers are located adjacent to the tumor bed. Five-year outcomes of three-dimensional, conformal, external beam radiation therapy using accelerated partial breast irradiation therapy has shown no local recurrences and no significant differences in survival rates than seen with WBI therapy.9
- Internal partial breast irradiation therapy (brachytherapy) -- Brachytherapy (trade name of MammoSite Balloon Brachytherapy) is an internal delivery of irradiation therapy requiring the placement of a catheter into the lumpectomy cavity. Through the catheter, radiation is delivered to the region in and around the lumpectomy cavity. Results of a five-year study show similar results in recurrence and survival rates to WBI.10
A second study comparing long-term complications with WBI to MammoSite showed 27% of MammoSite patients had a palpable mass at the lumpectomy site as compared to 7% of the same finding in WBI patients; 24% of MammoSite patients had telangiectasia (spider veins) as compared to 4% of WBI patients.11
- Intraoperative radiation therapy -- Intraoperative radiation therapy (TARGIT) is beneficial to women with early Stage I invasive ductal carcinoma. At the time of the lumpectomy, radiation is delivered to the lumpectomy cavity, or six weeks later a reincision is made to deliver the radiation to the lumpectomy cavity. It is a one-time delivery of irradiation therapy.12
In the TARGIT study of 1,721 patients, half received intraoperative radiation at the time of the lumpectomy, and the other half received intraoperative therapy after the lumpectomy. TARGIT patients were compared with 1,730 WBI patients. Results of the study showed a five-year risk for local recurrence of 3.3% for TARGIT patients who received the radiation therapy after the lumpectomy compared to 1.3% for WBI patients (P = 0.042). TARGIT patients who had intraoperative radiation therapy at the time of the lumpectomy had recurrences of 2.1% compared with 1.1% of WBI patients (P = 0.31). Breast cancer mortality was similar between the groups (P = 0.5).13
What is Proton Therapy?
Proton therapy is a form of irradiation treatment that permits high doses of radiation to the tumor area while halting the dosage at the Bragg peak at the site as designated in the computerized plan. It prevents radiation from affecting surrounding healthy tissues and nearby organs such as the lungs and the heart.
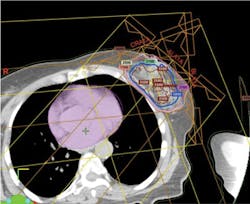
In very simplified terms, the treatment plan is accomplished by conducting computed tomography (CT) scans through the tumor area of the breast and the chest area. From this scan, a three-dimensional reconstruction of the tumor is created and transformed to a computerized image. The image is then used to plan the entrance of proton beams at multiple angles to the tumor bed area and also to determine intensity of dosage at each spot (beam scanning). Beam scanning, technically known as "pencil beam proton therapy" is a plan to "paint" a radiation dose layer by layer. Doctors and researchers at MD Anderson Cancer Center in Houston pioneered pencil beam proton therapy (see Figure 1).
For a detailed understanding of the nuclear principles of proton therapy, go to the MD Anderson link at http://www.mdanderson.org/patient-and-cancer-information/proton-therapy-center/what-is-proton-therapy/index.html.
The MD Anderson Center2 at the University of Texas describes proton therapy as a 196-ton, cancer-killing machine with submillimeter precision that can target a patient's tumor "while sparing nearby healthy tissues and minimizing side effects. In its most simple terms, that's proton therapy."
Proton vs. Photon Irradiation
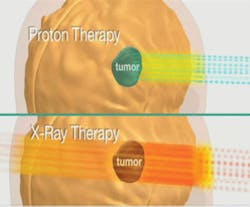
External-beam proton irradiation has a significant reduction in integral dose of radiation to the patient that is 60% lower than any external-beam photon (standard form of breast cancer irradiation therapy). The proton beam does not have an exit dose. It stops short at a pre-planned designated point just past the tumor bed (see Figure 2).
Radiologists, nuclear physicists, and dosimetricists create a computerized plan that controls the proton beam so that adjacent healthy tissue and organs are unaffected by the irradiation therapy. Planning of radiation distribution is part of both photon and proton treatment plans, but proton therapy can be delivered with great accuracy since proton beams can be controlled. The computerized plan is set to designate exactly where the beam starts and stops in relationship to the lumpectomy cavity.14,15
Since photon radiation always has an exit dose, it is impossible to completely avoid radiation from reaching surrounding healthy tissue and organs in left breast cancer.7 Proton irradiation therapy does not affect untargeted surrounding healthy tissues or nearby organs during proton-beam irradiation therapy, so patients do not experience fatigue and other side effects that are more common to photon irradiation therapy. Cosmetically, proton irradiation therapy will not cause the long-term effects of skin discoloration, leathery appearance, or hardening of the breast tissue (fat necrosis) that has been known to occur with photon therapy.15,16,17
Several clinical trials have shown excellent results with accelerated partial breast proton irradiation in patients with early breast cancer. Survival rates and disease recurrence rates are the same as those seen with photon partial breast irradiation or WBI.15,16,17,18
In a phase II clinical trial conducted at Loma Linda University Medical Center, 50 early breast cancer patients who had invasive (nonlobular) tumors (< 3cm) were treated with lumpectomy and partial breast proton irradiation therapy. The treatments occurred once a day for 10 days. The five-year follow-up showed over 90% of patients breast cancer free with close to 100% overall survival. More than five years after the completion of treatment, only one patient had recurrent cancer in the breast that was treated. All of the other patients completed the treatment without any side effects, and only three patients had skin telangiectasia at the five-year follow-up. An analysis showed near-complete elimination of radiation dose to the contralateral breast, lung, and heart.16
Another phase II clinical trial conducted at the National Cancer Center in Seoul, Korea, included 30 patients who had accelerated partial proton irradiation therapy once a day for five days. Half of the patients were treated with single-field proton-beam irradiation, and the other half were treated with two-field proton beam. All of the patients were alive at the 59-month follow-up and none of them had recurrent cancer in the breast treated. In those treated with single-field proton therapy, there were some mild to moderate cases of dermatitis, and one patient with severe wet desquamation. The patients treated with two-field proton-beam irradiation had good to excellent cosmetic results.18
Proton Therapy Expense
There are currently only 11 proton therapy centers in the United States with eight more in the construction phase. The cost to build a proton therapy center could be over 200 million dollars. The cost to fund clinical trials to provide evidence that proton therapy is superior to photon therapy is an additional expense that raises costs for patient treatment. As a result, the cost of treatment for proton therapy is twice that of photon irradiation therapy. Medicare and some insurance companies may pay for proton therapy.19
However, the proton therapy benefits of no treatment side effects, excellent cosmetic results, and prevention of secondary cancers and heart and lung disease are priceless!
Dr. Eric Strom is the medical director in the department of radiation oncology at Nellie B. Connally Breast Center, which is part of the MD Anderson Cancer Center in Houston. He has conducted numerous breast cancer treatment studies with his colleagues at MD Anderson. Currently, Dr. Strom is leading a clinical trial in accelerated partial breast irradiation proton therapy for early breast cancer. In a recent interview, Dr. Strom stated, "Each breast cancer patient should have an individualized treatment plan specific to their needs; no one plan fits all patients."
If you or someone you know is diagnosed with early breast cancer, get several opinions concerning treatment options. There is time to conduct the research. Consider clinical trials and search for them through this link http://clinicaltrials.gov/. Lastly, please place this article in the reception area of your office or clinic to share with patients. RDH
CYNTHIA BIRON LEISECA is president of DH Methods of Education, Inc., Home of Boot Camp for Dental Hygiene Educators. She is also the producer of two DVDs, "Precision in Periodontal Instrumentation," and "A Focus on Fulcrums." Cynthia is the distributor of "The Sharpening Horse Kit," www.DHmethEd.com.
References
1. Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Cancer. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. 2013 Apr 1;119(7):1402-11. doi: 10.1002/cncr.27795. Epub 2013 Jan 28.
2. Women with breast cancer may overestimate secondary risks. http://www.nlm.nih.gov/medlineplus/news/fullstory_140724.html.
3. Narod SA. The impact of contralateral mastectomy on mortality in BRCA1 and BRCA2 mutation carriers with breast cancer. Breast Cancer Res Treat. 2011 Jul;128(2):581-3. doi: 10.1007/s10549-011-1479-1. Epub 2011 Apr 1.
4. Chen S, Parmigiani G. J Clin Oncol. Meta-analysis of BRCA1 and BRCA2 penetrance. 2007 Apr 10;25(11):1329-33. Departments of Environmental Health Sciences and Biostatistics, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21205, USA. [email protected].
5. Burger AE, Pain SJ, Peley G. Treatment of recurrent breast cancer following breast conserving surgery. Breast J. 2013 May-Jun;19(3):310-8.
6. American Cancer Society. Radiation for breast cancer. Accessed 12/19/2013.
7. Brenner DJ, Shuryak I, Jozsef G, DeWyngaert KJ, Formenti SC. Risk and Risk Reduction of Major Coronary Events Associated with Contemporary Breast Radiotherapy. JAMA Intern Med. October 28, 2013.
8. Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. The Lancet Oncology. 2013; 14(11): 1086 – 1094.
9. Rodríguez N, Sanz X, Dengra J, Foro P, Membrive I, Reig A, Quera J, Fernández-Velilla E, Pera O, Lio J, Lozano J, Algara M. Five-year outcomes, cosmesis, and toxicity with 3-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2013 Dec 1;87(5):1051-7. doi: 10.1016/j.ijrobp.2013.08.046. Epub 2013 Oct 22.
10. Vargo JA, Verma V, Kim H, Kalash R, Heron DE, Johnson R, Beriwal S. Extended (5-year) outcomes of accelerated partial breast irradiation using MammoSite Balloon Brachytherapy: Patterns of failure, patient selection, and dosimetric correlates for late toxicity. Int J Radiat Oncol Biol Phys. 2013 Nov 21. pii: S0360-3016(13)00632-9. doi: 10.1016/j.ijrobp.2013.05.039.
11. Rosenkranz KM, Tsui E, McCabe EB, Gui J, Underhill K, Barth RJ Jr. Increased rates of long-term complications after MammoSite brachytherapy compared with whole breast radiation therapy. J Am Coll Surg. 2013 Sep;217(3):497-502. doi: 10.1016/j.jamcollsurg.2013.03.028. Epub 2013 Jul 2.
12. Ruano-Ravina A, Cantero-Muñoz P, Eraso Urién A. Efficacy and safety of intraoperative radiotherapy in breast cancer: a systematic review. Cancer letter. 2011 Dec 26;313(1):15-25. doi: 10.1016/j.canlet.2011.08.020. Epub 2011 Aug 31.
13. Elsberger B, Romsauerova A, Vinnicombe S, Whelehan P, Brown DC, Dewar JA, Thompson AM, Evans A. Eur J Surg Oncol. Comparison of mammographic findings after intraoperative radiotherapy or external beam whole breast radiotherapy. 2013 Dec 4. pii: S0748-7983(13)00919-0. doi: 10.1016/j.ejso.2013.11.011.
14. DeLaney TF. Proton therapy in the clinic. Front Radiat Ther Oncol. 2011;43:465-85. doi: 10.1159/000322511. Epub 2011 May 20.
15. Wang X, Amos RA, Zhang X, Taddei PJ, Woodward WA, Hoffman KE, Yu TK, Tereffe W, Oh J, Perkins GH, Salehpour M, Zhang SX, Sun TL, Gillin M, Buchholz TA, Strom EA. External-beam accelerated partial breast irradiation using multiple proton beam configurations. Int J Radiat Oncol Biol Phys. 2011 Aug 1;80(5):1464-72. doi: 10.1016/j.ijrobp.2010.04.052. Epub 2010 Aug 12.
16. Bush DA, Slater JD, Garberoglio C, Do S, Lum S, Slater JM. Partial breast irradiation delivered with proton beam: results of a phase II trial. Clin Breast Cancer. 2011 Aug;11(4):241-5. doi: 10.1016/j.clbc.2011.03.023. Epub 2011 Jun 12.
17. Wang X, Zhang X, Li X, Amos RA, Shaitelman SF, Hoffman K, Howell R, Salehpour M, Zhang SX, Sun TL, Smith B, Tereffe W, Perkins GH, Buchholz TA, Strom EA, Woodward WA. Accelerated partial-breast irradiation using intensity-modulated proton radiotherapy: do uncertainties outweigh potential benefits? Br J Radiol. 2013 Sep;86(1029):20130176. doi: 10.1259/bjr.20130176. Epub 2013 May 31.
18. Chang JH, Lee NK, Kim JY, Kim YJ, Moon SH, Kim TH, Kim JY, Kim DY, Cho KH, Shin KH. Phase II trial of proton beam accelerated partial breast irradiation in breast cancer. Radiother Oncol. 2013 Aug;108(2):209-14. doi: 10.1016/j.radonc.2013.06.008. Epub 2013 Jul 25.
19.Suit H, Kooy H, Trofimov A, Farr J, Munzenrider J, DeLaney T, Loeffler J, Clasie B, Safai S, Paganetti H. Should positive phase III clinical trial data be required before proton beam therapy is more widely adopted? No. Radiother Oncol. 2008 Feb;86(2):148-53. doi: 10.1016/j.radonc.2007.12.024. Epub 2008 Jan 30.
Past RDH Issues