Disposable, and sterile too: One company ensures sterile disposable prophy angles
By Lory Laughter, RDH, BS, MS
Much has changed about infection control in the dental practice during my 21 years of practice. While I don't date back to the days before gloves, cloth bags were used during sterilization until the middle of my junior year. Each setup was placed in a plastic basket, and two or three baskets were then put inside a cloth bag. Once sterilized, the plastic baskets were placed in our unit drawers-yes, uncovered.
One day was devoted to a new sterilization method in our clinic. Baskets were placed in sealed bags and left in the bags until opened for patient care. Today, no one would ever imagine storing instruments in a drawer outside a sealed bag. Yet, there was a time when it was a common and accepted practice.
Protocols changed and patients were further protected from potential harm. Health care is not a stagnant profession. Practices learned in school today will not be the protocols of tomorrow. With increased knowledge comes change beneficial to practitioner and clients alike.
Miscommunication About Infection Control
Infection control is one area where questions abound. It is difficult to find a day on social media or dental related sites where a query on proper infection control is absent. The concepts of decontamination, disinfection, and sterilization are often interchanged and misinformation ensues. While all three terms do apply to actions employed to protect the health and safety of dental patients as well as professionals, they are very different in meaning.
Figure 1: Pac-Dent's Sterile Mini 90 and Sterile Mini Ergo
Decontamination is a broad term and means an area, device, item or material is made safe to handle.1 The goal is to reduce the level of microbiological contaminates so there can be no transmission of infection. While decontamination is most often used in reference to radiation, it occurs in most settings. At a restaurant, the table is wiped between patrons. Many grocery stores now provide wipes for cart handles, and even some airlines periodically replace the headrest cover. These are all examples of decontaminating an area.
There are few uses for decontamination in dentistry other than cleaning a waiting room or removing the garbage regularly. One can think of decontamination in the dental setting as cleaning.
Disinfection removes nearly all pathogenic microorganisms from inanimate objects, but not necessarily all forms of the pathogens. For example, disinfection may not remove or kill spores.2 As we know, there are levels of disinfection; but, in general, these levels are not enough overkill of microorganisms to ensure no infectious material remains.
In dentistry, we disinfect surfaces and items that do not contact blood or mucus membranes. Keep in mind that any disinfectant used in the office must be an EPA-approved hospital disinfectant that is also tuberculocidal. The product must also be used according to label directions.
This brings us to sterilization. Sterilization is an absolute and is achieved only when a device, item, or surface is completely free of living microorganisms and viruses.2 Once microorganisms or viruses are reintroduced to the environment, sterilization no longer exists.
Instruments are not sterile the entire time we use them, but they do need to be microorganism-free prior to touching the patient's oral tissue. You do not want to transfer pathogens from a surface or one person to another. Sterilization can be achieved by a number of means including heat, chemical gases, ozone, and radiation.
Are disposable items sterile?
This review leads to a very important point of question. If items that come into contact with mucosal tissues are to be sterile, why are single-use disposable items used in dentistry not normally sterilized? I've worked in practices where 2x2 gauzes were sterilized prior to use in surgical situations. When gauze is used in the oral cavity, it makes sense that it should be sterile, not introducing microorganisms with the potential to cause infection.
Yet, unsterile single-use burs, saliva ejectors, lip retractors, and prophy angles are the norm.
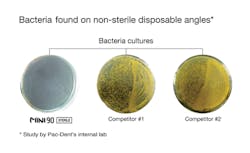
A study published in The Dental Advisor (March 2012) demonstrated unsterile pre-packaged burs showed bacterial colony growth when cultured on blood agar plates. In contrast, pre-sterilized burs showed no bacterial growth. During the study, burs were exposed to a broth containing 5% sheep's blood for 30 seconds. Unsterile pre-packaged burs, pre-sterilized packaged burs, and previously used burs sterilized by autoclaving were tested. The autoclaved burs served as a control. The study concluded that contamination of pre-packaged burs is possible and infection control can be maintained by using pre-sterilized burs (see Figure 2).3
Pre-packaged prophy angles also come into contact with mucosal tissues. The U.S. Food and Drug Administation does not require disposable prophy angles to be sterilized prior to packaging. Although prophy angles are manufactured in controlled environments subject to inspection and testing of equipment, there is no requirement of sterility. Just like the manufacturing of burs, the manufacturing of disposable prophy angles simply occurs in environments designed to minimize contamination.
Figure 2: Petri dish images of colonies
Sterile disposable prophy angles
These same angles come into contact with oral tissue during treatment where blood may be present and microorganisms can be harmful. It makes sense to protect the patient by using devices inside the oral cavity that are sterile.
Pac-Dent manufactures disposable, single use prophy angles which are sterilized after packaging. The angles are gamma-irradiated and guaranteed sterile. Considering the potential for tissue trauma during scaling, a sterile disposable prophy angle can help promote a safer healing condition. It is finally possible to have the convenience of a single-use item and the peace of mind that the angle is as sterile as an autoclaved metal angle.
It is worth mentioning that the disposable prophy cups that accompany the use of autoclaved metal angles are not sterilized. Under independent laboratory testing, the surfaces of Pac-Dent's Sterile Mini disposable prophy angles were shown to be completely free of pathogens.
The Sterile Mini 90 and Mini Ergo disposable prophy angles (DPA) from Pac-Dent have several advantages. Beyond sterilization, the angles are smooth running and quiet. They also have the smallest head, which increases visibility and provides for improved access. The gear and drive shaft technology from Pac-Dent has been proven in performance. The angles are 100% latex free.
The Mini DPAs have a beveled gear system shown to be more durable than the standard block gear format. This beveling provides for quiet and chatter free operation. The cup is small for a more functional reach. The benefits of these angles are evident in convenience, smooth running gears, improved visibility and enhanced infection control (see Figure 1).
Dentistry is an ever-changing profession. Improvements in techniques, instruments, procedures, and especially infection control need to be embraced and implemented. Patient safety is paramount to what we do every day. It makes sense to do everything with our power to decrease or eliminate introducing potentially harmful pathogens to those who trust us with their care.
I see a future for gamma-irradiated single-use products in dentistry. Look around your operatory work environment. You will notice other items that come into contact with the oral cavity but are not sterile. Saliva ejectors, fluoride trays, cotton rolls, and cotton roll holders are all potentially contaminated items common during dental hygiene treatment. Imagine the peace of mind possible in telling your patients that everything you put in their mouth is pathogen free.
Yes, this requires a change in our thought process. No longer will we be able to accept the possibility of disease transmission from unsterile items. Emphasis needs to be placed on heightening patient safety without extensive inconvenience or cost to the practice. Sterilization of single-use and pre-packaged products by gamma-Irradiation can provide that assurance.
You would never dream of using an unsterile curette on a patient; hold that same standard for your chosen prophy angle. RDH
Lory Laughter, RDH, BS, MS, practices clinically in Napa, Calif. She is owner of Dental IQ, a business responsible for the Annual Napa Dental Experience. Lory combines her love for travel with speaking nationally on a variety of topics. She is also a part-time educator or consultant for American Eagle, Livionex, and Nuvora. She can be contacted at [email protected].
References
1. www.cdc.gov/biosafety/publications/bmbl5/BMBL5_appendixB.pdf pg 329.
2. www.cdc.gov/biosafety/publications/bmbl5/BMBL5_appendixB.pdf pg 327.
3. Molinari JA, Nelson P. Research Report, The Dental Advisor Issue 45, March 2012.