Applications for dentifrice are for caries prevention and a whole lot more
By Kimberly Bray, RDH, MS
What determines your level of confidence in recommending a product to your patients? My confidence level depends on doing some of my own research and coming to my own conclusions.
---------------------------------------------------------------------------
More articles
- RDH hosts panel discussion about oil pulling
- Cheat sheet for periodontal protocol
- 4 ways to improve communication with specialty dental practices
---------------------------------------------------------------------------
When I first heard about Crest Pro-Health toothpaste and the wide range of cosmetic and therapeutic benefits it provides, I have to admit I was curious. The only product I own that can do just about anything is my smartphone! It's a phone, camera, iPod, and so much more! Then I started wondering, what if toothpaste could work that way, providing all of the key oral health benefits in a single tube? What would be the advantages? As it turns out, I could think of quite a few:
- Convenience. Many patients just don't have time to use more than one oral care product to get a wide range of benefits. They would prefer to simply use one product.
- No trade-offs. Patients could get therapeutic benefits without trading off cosmetic benefits of extrinsic whitening, tartar control, and breath protection.
- No selection required. Unlike my smartphone, where I select the app I want, a multi-benefit dentifrice would provide all of the benefits with each use.
- Widely applicable. It would be a product that would offer benefits for both teens and adults alike.
- Provide therapeutic protection. It would provide protection against caries, plaque, gingivitis, and sensitivity.
My list of potential benefits turned out to be pretty impressive. So I decided to do some research on Crest Pro-Health toothpaste. Here are the questions I asked and what I learned.
What is the basis for Crest Pro-Health formulations?
Crest Pro-Health (CPH) dentifrice is based on a unique, patented system of stabilized stannous fluoride (SnF2) and a cosmetic ingredient, sodium hexametaphosphate (NaHMP). Stannous fluoride has a long history of use in oral products for protection against caries, sensitivity, plaque, gingivitis, and oral malodor.1 Crest with Fluoristan, introduced by Procter & Gamble (P&G) in 1955, contained SnF2 and was the first dentifrice to receive the American Dental Association (ADA) Seal of Acceptance for the therapeutic prevention of caries. Stannous fluoride is the only fluoride source to provide benefits against caries, sensitivity, and plaque/gingivitis. It was the potential of this multi-benefit therapeutic agent that motivated P&G scientists to work for more than three decades to overcome the early limitations of SnF2-based dentifrices. These limitations included formula stability, an astringent taste, and mild extrinsic staining of teeth in some patients.
One breakthrough along the way was the discovery of polyphosphates, such as NaHMP, as cosmetic agents. Pyrophosphates were used in Crest Tartar Control dentifrices to provide tartar control benefits. Compared to pyrophosphate, NaHMP is a larger polymer with more potential attachment sites to the tooth surface. This larger size improves adsorption to tooth enamel, which provides surface stain removal and interferes with calcification of plaque to provide tartar control benefits. NaHMP was successfully used in Crest toothpastes to improve whitening benefits. The successful formulation of NaHMP and stabilized SnF2 in a single dentifrice formulation is the key breakthrough leading to the introduction of CPH dentifrice in 2005.
How does CPH dentifrice perform?
CPH dentifrices containing a system of stabilized SnF2 and NaHMP have been shown to provide a full range of therapeutic and cosmetic benefits (see Figure 1). The efficacy of CPH dentifrice has been demonstrated in randomized, blinded, controlled, and independent clinical studies.
Based on these clinical studies, CPH dentifrice has been awarded the Seal of Acceptance from the ADA in five categories: cavities; gingivitis and plaque; oral malodor; sensitivity; and whitening. In fact, CPH dentifrice is the only toothpaste on the market to earn acceptance in all five categories.
Efficacy demonstrated in technical studies, clinical trials
Over 80 publications and research presentations support the efficacy of CPH dentifrice. The results show CPH dentifrice is:
1. Effective in preventing and reducing the incidence of caries. Use of a fluoride-containing dentifrice is known to be effective in reducing caries and reversing early carious lesions by promoting remineralization and preventing demineralization.2
In addition, fluoride may also limit the production of acid associated with cariogenic bacteria.3 Stookey et al. conducted a two-year clinical trial with 955 subjects. A dual-phase prototype of CPH provided 17% to 25% fewer caries relative to a standard sodium fluoride (NaF) dentifrice.4 Anticaries benefits were also demonstrated by Wefel et al. in an in situ study.5
2. Effective in building protection against dentinal hypersensitivity. Laboratory studies show SnF2 reacts to form precipitates, which occlude dentinal tubules and provide sensitivity relief. Figure 2 shows high magnification scanning electron micrographs (SEM) of dentinal tubules before and after the use of CPH dentifrice.1
Independent clinical studies showed significant fast6,7,8 and long-term sensitivity relief as measured by tactile and thermal methods compared to standard fluoride negative controls. Results from one clinical study showed a 44% decrease in thermal sensitivity and up to a two times greater tolerance to tactile sensitivity after eight weeks of use.6
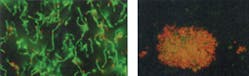
3. Effective in reducing plaque and gingivitis. These benefits are due to the broad spectrum antibacterial action of SnF2. By killing and inhibiting bacteria associated with plaque, CPH dentifrice reduces the development of gingivitis. Gingivitis, if left untreated, can lead to periodontitis, which can eventually lead to tooth loss. Emerging research suggests that poor gingival health may be linked to systemic conditions.9 Figure 3 demonstrates that the antibacterial activity of CPH dentifrice remains strong for 16 hours compared to a control in a live/dead assay.10
Numerous clinical studies, ranging from short-term studies to six-month clinical trials, have shown significant reductions in plaque, gingival inflammation, and bleeding after use of CPH dentifrice relative to positive and negative controls.11-17
4. Effective in reducing breath malodor. The antibacterial action of SnF2 inhibits the breakdown of residual proteins in the mouth to form volatile sulfur compounds responsible for oral malodor.18 Two independent clinical studies involving a total of 75 subjects showed significant reductions in halitosis overnight after using CPH dentifrice compared to a standard NaF control.19
A longer-term study20 of 71 subjects showed significant reductions in halitosis after one week and three weeks of CPH use compared to a standard NaF control.
5. Effective in reducing formation of calculus. Laboratory studies have shown that NaHMP significantly reduces the crystal growth and mineralization of plaque either in aqueous solution or in a dentifrice compared to a conventional anti-tartar dentifrice containing pyrophosphate.21 These findings were supported by results of two independent six-month clinical trials in which CPH dentifrice and a CPH prototype showed a 56% and 55% reduction in calculus formation, respectively, compared to marketed controls at six months.22,23
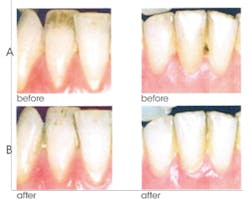
6. Effective in whitening teeth by removal and prevention of stains. The combined action of NaHMP and an advanced, high cleaning silica system results in stain removal and whitening benefits. The surface activity of NaHMP competes with stains for surface sites, effectively preventing the buildup of new stains. Figure 4 shows the removal of surface stains after only two weeks of CPH use.
Four separate clinical trials, summarized in two publications, compared the stain removal efficacy of CPH dentifrice with that of a positive control whitening dentifrice at two different time points: baseline and two weeks;24 and baseline, three, and six weeks.25 In all cases, a highly significant improvement in stain removal was measured from baseline for both the CPH and positive control whitening dentifrice. In addition, the whitening benefits of the CPH dentifrice were not significantly different from the positive control.
What do patients and professionals think about CPH?
The efficacy of CPH dentifrice is supported by an extensive body of clinical evidence. However, its success ultimately depends upon its effectiveness and acceptability to users in the home environment. The question is do the benefits measured or observed in a controlled clinical environment by clinical specialists translate into product acceptability? In other words, are dental benefits observed by patients and dental professionals in clinical studies evident when used in the home environment? These questions have been addressed in two recent home-use studies. These studies showed that CPH dentifrice is effective for and acceptable to both patients and dental professionals who used it at home as part of their normal oral hygiene routine.
Practice-based assessment. A practice-based assessment of CPH dentifrice was conducted among patients across the USA.26 In this study, both patients and their dental professionals answered a questionnaire at the beginning and at the end of the three- to four-month use period (up to six months in 25% of cases).
Of the 1,078 patients who responded:
- 88% rated the product "excellent/very good" or "good"
- 77% of those who noticed improvements in their oral health planned to continue using the product
- 83% rated CPH positively for reducing surface stains
- 9 out of 10 patients rated the product positively in the areas of "keeping mouth healthy," "cleaning teeth thoroughly," "making gums healthier," and "freshening breath" (see Figure 5).
Of the 1,267 responses from dental hygienists and dentists:
- 68% noted improvement in their patients' gingival bleeding/inflammation and a reduction in the formation of calculus
- 61% noted reduced sensitivity
- 57% noticed reduced staining
Eighty percent of the dental professionals indicated they would recommend CPH dentifrice to their other patients. That jumped to 91% of dental professionals who noted improvements in their patients' oral health or staining.
Usage study among dental professionals. Recently, a CPH usage experience study was conducted among dental professionals.27 After receiving a tube of Crest Pro-Health (Clinical Gum Protection variant) for their personal use, approximately 2,100 dental professionals completed an optional online survey about their experience using the product:
- 99% of dental professionals rated their experience with the product as "excellent/very good/good"
- 96% indicated they would continue to use the product
- 92% agreed that dentifrices containing SnF2 can benefit their patients more than other toothpastes
- 81% said they had recommended CPH dentifrice to patients in the past and 96% said they would recommend CPH to more patients now that they had experienced the product themselves (see Figure 6).
When asked why they would recommend CPH to more of their patients, some responses given were:
- "I believe patients can benefit from this product."
- "I can only vouch for a product I have personally used and liked."
- "I believe this is the best product on the market right now."
- "Ortho patients need that extra level of protection."
- "It feels clean, and there was noticeable plaque reduction in my mouth."
- "It helped with my sensitivity and has a nice, refreshing taste. It's also good for the gums."
- "I have seen a clinical improvement with Crest Pro-Health."
Clinical studies demonstrate that CPH dentifrice puts the power and convenience of an efficacious, multi-benefit toothpaste in the palm of your hand, providing seven therapeutic and cosmetic benefits with each use. Real-world, in-home studies show the efficacy of CPH dentifrice established in controlled clinical trials translates into effectiveness and acceptability among both patients and dental professionals.
Recent studies have shown that dental care routines that include CPH, an Oral-B oscillating-rotating power toothbrush, and regular use of dental floss can further enhance oral care benefits to patients.
These findings show that you can be confident in recommending CPH dentifrice to your patients, knowing that the vast majority are likely to notice and appreciate benefits of a clean, healthy mouth and gums.
Author's acknowledgment: To Ms. Anita Guy for assistance with manuscript preparation.
KIMBERLY BRAY is professor and director for the Division of Dental Hygiene at the University of Missouri-Kansas City School of Dentistry. She currently teaches in three degree programs including two degrees with distance learning options. Prof. Bray has 24 years of clinical experience in both general and periodontal practice with research interests in patient adherence, alternative learning strategies, and product efficacy.
References
1. Baig AA , He T. A novel dentifrice technology for advanced oral health protection: a review of technical and clinical data. Compend Cont Educ Dent 2005;26 (supp 1):4-11.
2. Marinho VC, Higgins JP, Sheiham A, et al. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003;1 CD00278.
3. Hamilton IR. Effect of fluoride on enzymatic regulation of bacterial carbohydrate metabolism. Caries Res 1977;11:269-291.
4. Stookey GK, Mau MS, Isaacs RL, et al. The relative anticaries effectiveness of three fluoride-containing dentifrices in Puerto Rico. Caries Res 2004;38:542-550.
5. Wefel JS, Standford CM, Ament DK, et al. In situ evaluation of sodium hexametaphosphate-containing dentifrices. Caries Res 2002;36:122-128.
6. Schiff T, Saletta L, Baker RA, et al. Desensitizing effect of a stabilized stannous fluoride/sodium hexametaphosphate dentifrice. Compend Cont Educ Dent 2005;26 (supp 1):35-40.
7. Schiff T, He T, Sagel L, Baker R. Efficacy and safety of a novel stabilized stannous fluoride and sodium hexametaphosphate dentifrice for dentinal hypersensitivity. J Contemp Dent Pract 2006;May (7)2:001-008.
8. He T, Barker ML, Qaqish J, Sharma N. Fast onset sensitivity relief of a 0.454% stannous fluoride dentifrice. J Clin Dent 2011;22:46-50.
9. Guynup S. Our mouths, ourselves. Sci Am 2005;3-5.
10. Ramji N, Baig AA, He T, et al. Sustained antibacterial actions of a new stabilized stannous fluoride dentifrice containing sodium hexametaphosphate. Compend Cont Educ Dent 2005;26 (supp 1):19-28.
11. White DJ. A 24-hour dental plaque prevention study with a stannous fluoride dentifrice containing hexametaphosphate. J Contemp Dent Pract 2006;July (7)3:001-011.
12. Mankodi S, Bartizek RD, Winston JL, et al. Anti-gingivitis efficacy of a stabilized 0.454% stannous fluoride/sodium hexametaphosphate dentifrice: a controlled six-month clinical trial. J Clin Periodontol 2005;32:75-80.
13. Mallatt M, Mankodi S, Bauroth K, et al. A controlled 6-month clinical trial to study the effects of a stannous fluoride dentifrice on gingivitis. J Clin Periodontol 2007;23 epub.
14. Archila L, Bartizek RD, Winston JL, et al. The comparative efficacy of stabilized stannous fluoride/sodium hexametaphosphate dentifrice and sodium fluoride/triclosan/copolymer dentifrice for the control of gingivitis: A six-month randomized clinical study. J Periodontol 2004;75(12):1592-1599.
15. Archila L, He T, Winston JL et al. Antigingivitis efficacy of a stabilized stannous fluoride/sodium hexametaphosphate dentifrice in subjects previously nonresponsive to a triclosan/copolymer dentifrice. Compend Cont Educ Dent 2005;26 (supp 1):12-18.
16. Sharma NC. Plaque control evaluation of a stabilized stannous fluoride dentifrice compared to a triclosan dentifrice in a six-week trial. J Clin Dent 2013;24:31-36.
17. He T. Evaluation of anti-gingivitis benefits of stannous fluoride dentifrice among triclosan dentifrice users. Am J Dent 2013;26:175-179.
18. Tonzetich J. Production and origin or oral malodor: a review of mechanisms and methods of analysis. J Periodontol 1977;48(1):13-20.
19. Farrell S, Barker ML, Gerlach RW. Overnight malodor effect with a 0.454% stabilized stannous fluoride sodium hexametaphosphate dentifrice. Compend Cont Educ Dent 2007;28(12):658-662.
20. Nachnani S, La S, Lee S, et al. Oral malodor reduction with 3-week use of 0.454% SnF2 dentifrice. J Dent Res (AADR/IADR) 2008;87(Spec Iss B): Abstract 2864.
21. White DJ, Cox ER, Suszcynsky-Meister EM, et al. In vitro studies of the anticalculus efficacy of a sodium hexametaphosphate whitening dentifrice. J. Clin Dent 2002;13:33-37.
22. Schiff T, Saletta L, Baker RA, et al. Anticalculus efficacy and safety of a stabilized stannous fluoride/sodium hexametaphosphate dentifrice. Compend Cont Educ Dent 2005;26(suppl 1):29-34.
23. Winston JL, Fiedler SK, Schiff T, et al. An anticalculus dentifrice with sodium hexametaphosphate and stannous fluoride: a six-month study of efficacy. Compend Cont Educ Dent 2007;Jul 1 8(5):1-8.
24. Terezhalmy G, Chaves E, Bsoul S, et al. Clinical evaluation of the stain removal efficacy of a novel stannous fluoride and sodium hexametaphosphate dentifrice. Am J Dent 2007;Feb 20(1):53-8.
25. He T, Baker R, Bartizek RD, et al. Extrinsic stain removal efficacy of a stannous fluoride dentifrice with sodium hexametaphosphate. J Clin Dent 2007;18(1):7-11.
26. Sensabaugh C, Sagel ME. Stannous fluoride dentifrice with sodium hexametaphosphate: Review of laboratory, clinical and practice-based data. J Dent Hyg 2009;83(2):70-8.
27. Data on file, Procter & Gamble, 2013.
Past RDH Issues