Periodontal Regeneration
A review of periodontal attachment and guided tissue regeneration.
This article is the conclusion of a two-part continuing education series presented by RDH. To obtain credit for reading this article, answer the quiz on page 37 and follow the instructions on the envelope inserted between pages 30-31. The first part of the series was printed in the October 1997 issue of RDH.
RDH`s continuing education program
Timothy J. Hempton, DDS,
Esther Wilkins, DMD, and
Diane Lancaster, RDH, BS
In the course of clinical practice, dental hygienists encounter many patients with various conditions of periodontal breakdown. Loss of attachment appears as pocket formation, gingival recession (clinical root exposure), or as a combination of either of these anatomical alterations.
Maintenance of periodontal pockets can be a daunting task if the pockets are deep and the involved root surfaces have deep grooves, concavities, or furcation exposure. Today, the potential for reversal of attachment loss exists. The reversal can lead to a reduction of probing depths, facilitating maintenance by the dental hygienist. Reversal of attachment loss through the development of a new attachment apparatus is known as guided-tissue regeneration. The purpose of this paper is to review the anatomy of the attachment apparatus and to describe the biology of guided-tissue regeneration (GTR).
Anatomy of the attachment apparatus
Normal anatomy of the periodontal-attachment apparatus consists of cementum, periodontal ligament (PDL), bone, and gingiva. The alveolar bone which encases the root does not interface with the root surface directly. Engagement of the root surface by the bone is accomplished through a series of biologic connecting cables - the fibers of the periodontal ligament. These biologic, connecting collagen cables extend from the alveolar bone toward the root surface. The periodontal ligament fibers are produced by fibroblasts, connective tissue cells found throughout the body. The fibroblasts of the PDL function specifically to maintain the fiber system which connects the tooth to the alveolar bone.
The fibers of the periodontal ligament terminate in a specialized tissue covering the root surface, the cementum. Cementum is similar to bone tissue and consists of living cells and a mineralized noncellular component. Connective tissue fibers, the Sharpey`s fibers, emerge from the cementum and engage the periodontal ligament fibers which extend from the alveolar bone.
The periodontal ligament fibers which make up the system not only connect the cementum-coated root surface to the alveolar bone but also act as a shock absorber for the tooth when it is under occlusal stress during function such as chewing food or parafunctional movements such as grinding of teeth or bruxing.
The cementum and fibroblasts of the periodontal ligament are all derived from the same source. The progenitor cells differentiate into cells which produce tissue components of the periodontal ligament, i.e., the periodontal ligament fibers and the periodontal ligament fibroblasts. The progenitor cells also differentiate into cementoblasts. These cells develop into cementocytes which produce cementum.
The biology of guided-tissue regeneration
The progenitor cell of the periodontal ligament is the focal point in the principle of guided-tissue regeneration. The technique of GTR affords unimpeded development and movement of the progenitor cells toward the root surface which had previously undergone attachment loss due to periodontal disease. It is critical that the root surface be properly debrided of all bacteria and related toxins for GTR to result in a new attachment apparatus.
The term "guided-tissue regeneration" refers to the facilitated movement of the progenitor cells toward the treated root surface with exclusion of gingival epithelial cells and fibroblasts. The cells normally would be impaired from migrating onto the root surface by the downgrowth of gingival epithelial cells. When a gingival flap is surgically elevated to access a defect, wound closure is predominantly achieved by a downward movement (apical migration) of gingival epithelial cells. The cells adhere to the root surface resulting in wound closure through a long junctional epithelial attachment that does not resemble the original attachment apparatus of periodontal ligament fibers. Instead, epithelial cells line the root surface and adhere through sticky cell surface components. The cells can and sometimes do detach, resulting in the redevelopment of a pocket.
During healing, both the gingival epithelial cells of the periodontal ligament and the progenitor cells migrate into the surgerized area to cause wound closure. The epithelial cells, however, develop and migrate at a faster rate than do the progenitor cells of the periodontal ligament. If epithelial cells can be excluded from a healing wound for a period of six to eight weeks, progenitor cells from the periodontal ligament have adequate time to enter the wound. Under normal circumstances, gingival epithelial cells would win the race between the two cell lines, resulting in a long junctional epithelial attachment as previously mentioned.
The exclusion of epithelial cells through GTR affords the progenitor cells of the PDL the opportunity to effect a wound closure, thereby limiting the apical extension of the gingival epithelium. The exclusion of the gingival epithelial and connective tissue is achieved by placement of a barrier membrane over the root surface and a portion of the exposed bone.
Once the progenitor cells proliferate into the defect they differentiate into cementocytes and fibroblasts. The cementocytes produce new cementum. The fibroblasts then produce the fibers of the periodontal ligament. Eventually, the previously diseased root surface, which was debrided during the surgical procedure, is covered with new cementum. The new PDL fibers engage the new cementum to the alveolar bone.
The process just described is how the development of a new attachment apparatus through GTR effects pocket closure.
As a technique, GTR is associated with osseous graft procedures. Utilization of GTR in theory results in periodontal regeneration of the attachment apparatus (i.e., new cementum and PDL) but doesn`t necessarily result in new bone.
Placement of osseous grafts in conjunction with GTR often is employed to enhance the development of new bone (bone regeneration). A bone graft could be obtained from the same patient - for example, a healing extraction socket or from an edentulous area. This is an autogenous graft (graft from the same individual). Another option would be to obtain cadaver bone from a tissue bank. The human bone graft material is freeze dried and sterilized. This type of graft is an allograft graft (graft material from the same species).
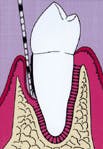
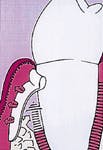
Figure one represents an intrabony defect. Figure two illustrates the same defect after soft-tissue flap elevation and root debridement. Wound healing is determined by a race between four cell lines. The four sources include gingival epithelium, progenitor cells from the periodontal ligament, gingival connective tissue, and bone. Figure three represents the wound with a membrane placed to facilitate guided-tissue regeneration.
The membrane can be resorbable or nonresorbable. Commercially available nonresorbable membranes are made of expanded polytetrafluorethylene (e-PTFE). A nonresorbable membrane must be removed in a second-stage surgical procedure. Resorbable membranes can be fabricated from collagen, polylactic acid, or vicryl mesh. A resorbable membrane will begin to degrade in six to eight weeks. As previously described, the purpose of the membrane is to serve as a barrier to exclude cells from the gingiva. This exclusion of cells, which migrate faster than progenitor cells from the PDL, provides adequate time for the PDL progenitor cells to migrate into the surgically treated area.
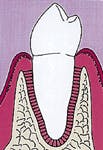
The PDL progenitor cells eventually develop or differentiate into two cell lines - cementocytes and periodontal ligament fibroblasts. The two cells produce a new attachment apparatus resulting in a wound closure, which, in principle, resembles the attachment apparatus prior to the infective process of periodontal disease. Figure four represents the new attachment apparatus (regeneration).
Clinical Application of GTR
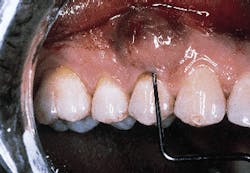
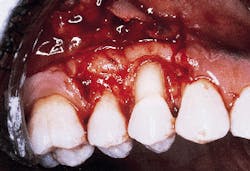
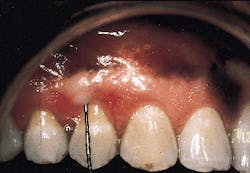
A patient with a periodontal abscess on the buccal of tooth #5 can be seen in photograph #1. Probing reveals a 6 mm pocket.
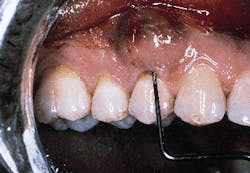
The defect subsequent to flap elevation can be seen in photograph #2. Calculus is evident on the buccal root surface.
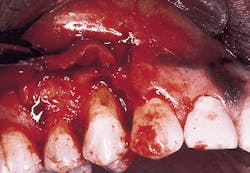
Photograph #3 is a view of a defect after thorough debridement. The periodontal disease process has resulted in an osseous dehiscence which extends 6 mm from the cementoenamel junction to the osseous crest on the mid-facial aspect of the involved premolar.
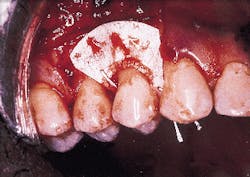
A nonresorbable membrane for GTR was placed over the defect as seen in Photograph #4. The membrane was composed of e-PTFE and was obtained from the W.L. Gore Co. in Flagstaff, Ariz.
The gingival flaps were repositioned covering the membrane on the buccal surface. In the case presented in this paper, no osseous grafting materials were utilized. The patient declined utilization of freeze-dried bone graft or harvesting of bone from another location in his mouth.
Photograph #5 was taken at the one-year postoperative visit. Probing depths around tooth #5 were reduced to 2 mm. Pocket closure was achieved and was maintained for a five-year observation period.
In principle, pocket closure in this case was obtained by regeneration of a new attachment apparatus.
GTR, when successful, results in the development of new cementum on the treated root surface which engages a newly developed periodontal ligament complex (fibers and fibroblasts of the periodontal ligament). By re-establishing the attachment apparatus on the previously diseased root surface, pocket reduction to probing depths of 3 mm or less can occur. This goal is an important objective as maintenance is facilitated, and the long-term prognosis for the involved tooth is improved.
References appeared with first part in October 1997 issue.
Timothy J. Hempton, DDS, and Esther Wilkins, RDH, DMD, are members of the department of periodontology at Tufts University School of Dental Medicine. Diane Lancaster, RDH, is a practicing hygienist in Dedham, Mass.
Photograph #1: An abscess is present on the facial aspect of tooth #5. Probing indicated a 6mm pocket.
Photograph #2: Subsequent to flap elevation a large piece of calculus is observed on the facial aspect of tooth #5.
Figure 1: A pocket is adjacent to an intrabony defect.
Figure 2: Subsequent to flap elevation and debridement of the root surface, wound healing ensues. Closure of the wound can be accomplished by gingival epithelium (1), gingival connective tissue (2), bone or (3) primary healing cells from the periodontal ligament (4). Usually healing is achieved through development of a long junctional epithelium.
Figure 3: A membrane (resorbable or nonresorbable) can be used to exclude gingival epithelial cells and connective tissue cells. This allows primary healing cells from the periodontal ligament to enter the wound and effect development of a new periodontal attachment apparatus.
Figure 4: This illustration demonstrates complete regeneration subsequent to utilization of a guided-tissue regeneration procedure. The bone loss which was present in Figure 1 has been restored with new bone, which engages the root surface through a new attachment apparatus.
Photograph #3: This photograph demonstrates the root surface subsequent to degranulation and root debridement. Hand instruments and ultrasonic scalers were employed to detoxify the root surface.
Photograph #4: An expanded polytetrafluorethlyne membrane was placed over the root surface and secured. The facial flap was then sutured over the membrane.
Photograph #5: The area one year postoperatively. Probing depths are reduced to 2mm. In principle, pocket elimination has been effected by development of a new attachment apparatus.
Quiz for periodontal regeneration article
1. In a healthy periodontium, alveolar bone is attached to the root via:
a. The periodontal ligament (PDL)
b. Ankylosis
c. A long junctional epithelium
d. None of the above
2. In a healthy periodontium, the root surface is covered with a layer known as:
a. Periostium
b. Granulation tissue
c. Cementum
d. None of the above
3. Which fibers emerge from the cementum to engage PDL fibers?
a. Wilkins fibers
b. Junctional fibers
c. Sharpey`s fibers
d. Polyactic acid fibers
4. The PDL functions as:
a. An attachment apparatus between the alveolar bone and the root
b. A shock absorber during occlusal function
c. Both A&B
d. None of the above
5. The function of a barrier membrane used during a GTR procedure is:
a. To exclude epithelium
b. To facilitate plaque retention
c. To reduce postoperative pain
d. None of the above
6. Subsequent to root debridement and closure of a gingival flap with sutures, which cells are the first to migrate apically and effect wound closure?
a. Fibroblasts
b. Epithelial cells
c. Osteocytes
d. None of the above
7. An e-PTHE (expanded polytetrafluoroethylene) membrane is:
a. Partially resorbable
b. Completely resorbable
c. Nonresorbable
d. None of the above
8. During a bone graft procedure, which type of barrier membrane can be used with an autogenous osseous graft?
a. A resorbable membrane
b. A nonresorbable membrane
c. Either A and B
d. None of the above
9. Subsequent to surgical treatment of an intrabony defect, progenitor cells from the PDL compete with epithelial cells and bone-forming cells to close the woun d. If epithelial cells win the race, what type of attachment results?
a. A long junctional epithelium
b. Regeneration of the PDL
c. A fibroblast adherence
d. None of the above
10. Which of the following statements regarding combined barrier membrane and bone graft placement for guided tissue regeneration is true?
a. A bone graft can be placed in conjunction with a barrier membrane
b. A bone graft should only be placed during the second stage surgery to remove a nonresorbable membrane
c. An allograft should never be placed in conjunction with a barrier membrane
d. None of the above
11. A therapist places an allograft with a collagen membrane. Which of the following statements is false?
a. The collagen membrane is resorbable
b. The membrane should facilitate development of PDL fibers to the regenerated bone
c. Collagen membranes are nonresorbable
d. Freeze-dried human cadaver bone is an allograft
12. Exclusion of cells other than progenitor cells from the PDL allows these cells to enter the wound and differentiate into:
a. Fibroblasts which produce a PDL attachment to the previously diseased root surface
b. Cementocytes which produce cementum covering the previously diseased root surface
c. Both A&B
d. None of the above
13. Which factor is important in obtaining regeneration of the periodontium on a diseased root surface?
a. Optimal debridement of the root surface of bacteria and related toxins
b. Exposure of one-half of the e-PTFE membrane immediately after surgery
c. Both A&B
d. None of the above
14. Which of the following barrier membranes must be removed in a second-stage procedure?
a. A collagen membrane
b. A polylactic acid membrane
c. A vicryl mesh mebrane
d. None of the above. These membranes are all resorbable
AADH
To receive continuing education credit for reading this article and answering the questions above, please use the answer sheet and business-reply card inserted betwen pages 30-31. Seventy percent of the questions must be answered correctly in order to receive certification for this course.