An Update on the Diagnosis and Prevention of Latex-Associated Allergic Reactions
A Peer-Reviewed Publication Written by Carol Ann Sims, DDS
Educational Objectives
After reading this course, the clinician will be able to do the following:
Introduction
The practice of healthcare has had many changes over the past century. Changes of historical note with respect to infection control were first recorded with the suggestions of Lister for guidelines on aseptic procedures. Other physicians suggested the practice of hand washing prior to medical students leaving autopsy suites before they entered the labor and delivery area of the hospital, as a means to decrease the transmission of infectious disease agents. As the HIV/AIDS epidemic was recognized in the United States in the early 1980’s, a multitude of new infection control measures swept the medical and dental community. The increased use of natural rubber latex products as an effective barrier for infection control practices presents the problem of a growing number of patients and healthcare workers with allergic reactions to products containing latex. There are several types of immune responses that occur with latex sensitivity, and certain populations are at greater risk. Products can be selected that decrease exposure to allergens and sensitivities in the dental office.
Latex Processing
William Halstead is the first person recognized for using latex surgical gloves, in 1890. It was nearly another century before routine use of gloves was recommended in dentistry in the middle of the 1980’s. This was a result of the introduction of universal precautions to provide protection from the transmission of blood-borne pathogens. In an effort to keep up with the increasing demand for gloves as a result of these recommendations, latex manufacturers had to increase production dramatically. It is estimated that tertiary-care facilities in the United States use over seven million pairs of gloves annually, and most dental offices will use approximately ten to twenty thousand pairs of gloves annually.1
Latex is produced from the rubber tree Hevea brasiliensis. Most of the supply of latex is from rubber trees grown in Malaysia, Indonesia, and Thailand.1,2,3 Latex is a milky sap that flows from these trees when workers make diagonal cuts into the bark. Less than 10% of the latex harvested is used for the manufacture of gloves. The latex rubber sap has a protein content of 15 mg/ml. Once the sap is collected, ammonia is added to prevent auto-coagulation and contamination of the latex.2,3 There are two concentrations of ammonia latex sold. High ammonia latex concentrate is 0.7% ammonia by weight, and low ammonia latex concentrate is 0.2% - 0.3% ammonia by weight. Higher levels of ammonia are used to stabilize the latex; however, higher levels of ammonia in examination gloves can cause skin irritations.4 For glove production, lower levels of ammonia are used to reduce skin irritation, but this requires the addition of accelerators and antioxidant preservatives.3,4
Insoluble latex proteins are responsible for the more severe allergic reactions experienced in persons with latex sensitivities. The addition of ammonia in glove processing reduces the latex protein content somewhat, but remaining proteins become incorporated and trapped into the gloves. Healthcare workers and patients with allergies to latex proteins should avoid latex product contact because of the severe reactions they could experience. There are four other causes of increased latex sensitivities. These involve the accelerators, additives, bleached cornstarch allergens, and latex allergens that are absorbed in powdered gloves.
Rubber used for glove manufacturing requires dipping. There are two techniques used for dipping. In the first method, ionic compounds and salts are deposited on formers that are used for dipping surgical gloves. The second method of dipping is called straight dipping. This technique is used for exam gloves. The formers are dipped directly into latex during straight dipping. The gloves are then oven-dried and passed through leaching tanks that remove water-soluble proteins and excess secondary preservatives.3 The heating process is called vulcanization. Vulcanization greatly enhances the strength, stability, and elasticity of the latex. Accelerators were added to the vulcanization step to decrease the amount of time and the temperatures required for processing. These accelerators are responsible for type IV allergic reactions experienced by healthcare workers and patients.3,4
Cow’s milk casein is sometimes added during the manufacturing of gloves. The addition of this compound improves the physical properties of the gloves. Ylitalo et al. demonstrated in a study of patients with a positive skin prick test to natural rubber latex allergies that false-positive results occurred as a result of casein present as a stabilizer in the glove. These false-positive patients were, in fact, allergic to milk and milk products rather than natural rubber latex.5
Gloves can be manufactured with powder or powder-free. Some manufacturers place powdered cornstarch into the gloves to make them easier to don. If the gloves are processed powder-free, they go through a separate processing method with the addition of a hydrogel polymer and chlorination to make them more slippery. It has been rare, but there are cases of cornstarch allergies reported in the literature.3,6 The addition of cornstarch powder causes an additional problem with latex proteins. The proteins are absorbed by the cornstarch, and each time the gloves are removed, aerosolized particles of latex protein become suspended in the air and can pose a problem for the latex-sensitive patient and healthcare worker.
Our immune system serves to protect the body from foreign materials. The immune system is responsible for the types of reactions that occur with latex sensitivity. In the first phase of the immune response, foreign antigens recognized by the body are mediated by B- and T-cell lymphocytes and macrophages. This process leads to the production of specific and nonspecific antibodies. Antibody production causes an increase in the inflammatory response system, and the final stage results in antigen destruction of foreign cells. Foreign cell antigens are destroyed by causing cell death or engulfing the antigen in the process of phagocytosis.
Latex-sensitivity Reactions
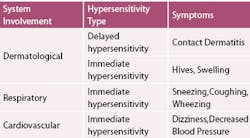
Signs and symptoms associated with latex allergens can involve the skin, respiratory tract, gastrointestinal system, and the cardiovascular system. (Table 1) Symptoms requiring immediate attention are hives, swelling, watering eyes, sneezing, coughing, wheezing, dizziness, and/or a decrease in blood pressure. Non-dermatological symptoms can be chronic sinusitis or asthma. Localized hand symptoms, such as itching, dermatitis, eczema, or vesicles, are dermatological signs.
There are three types of immune reactions that occur as a result of exposure to latex products (Table 2). The hypersensitivity reactions that occur as a result of natural rubber latex contact are irritant contact dermatitis, allergic contact dermatitis, and type I hypersensitivity reactions.2,3,4,7,8
Irritant Contact Dermatitis
Irritant contact dermatitis is a nonallergic inflammatory reaction that occurs as a result of damage to the protective layer of the skin. The clinical manifestations are dry, crusted lesions and itching. These symptoms are extremely similar to allergic contact dermatitis and can be ruled out by allergy testing. Eighty percent of the cases of contact dermatitis are a result of this nonallergic type of reaction. Preventing dry, chapped hands with moisturizers, minimizing contact with extremely hot water, and rinsing and drying hands thoroughly can reduce irritant contact dermatitis. Friction, perspiration, extreme temperatures, and exposure to soaps and detergents, rather than exposure to latex allergens, often cause irritant contact dermatitis in healthcare workers.2,3,4
Allergic Contact Dermatitis
Allergic contact dermatitis is a T-cell mediated immune response that generally occurs within two to four days following exposure to an allergen. This is considered a delayed reaction and is often referred to as a type IV hypersensitivity reaction. Type IV hypersensitivity reactions are more commonly caused by accelerators and antioxidants than by latex exposure. Since the clinical symptoms of allergic contact dermatitis - pruritis, dry skin, eczema, scabs, and vesicles - are similar to irritant contact dermatitis, it is necessary to confirm the allergic nature of the reaction in order to avoid further sensitization. Allergic contact dermatitis can develop after years of exposure to a substance.3
Immediate Allergic Response
Immediate allergic responses are considered type I immune hypersensitivity reactions. Clinical manifestations of type I latex hypersensitivity present as contact urticaria, rhinitis and asthma, or anaphylaxis. Immediate allergic reactions are IgE-mediated immune responses.
Contact Urticaria
Contact urticaria is the most common presentation of type I hypersensitivity reactions to latex. Itching, redness, and blistering occur within 15 minutes following contact exposure to latex. This reaction can occur as a result of type I hypersensitivity to cornstarch; however, this is a very rare occurrence.
Rhinitis and Asthma
Sneezing, watering eyes, nasal congestion, and an itching sensation of the oral palatal mucosa are clinical symptoms of a type I hypersensitivity reaction known as rhinitis, which is a result of mast cell degranulation and histamine release. Hyperinflation of the lungs with difficulty on expiration are the characteristic signs of asthma, which can be experienced by persons having a type I hypersensitivity reaction to latex.
Anaphylaxis
Anaphylactic hypersensitivity reactions are experienced within minutes of exposure to a specific antigen. Anaphylaxis is characterized by respiratory collapse and shock. Anaphylactic reactions that occur secondary to latex allergy most commonly arise intraoperatively and have been associated with allergen contact with mucosal surfaces. In dentistry, anaphylactic reactions to latex allergens have been reported to occur with exposure to gloves, dental rubber dams, and indirect exposure to latex glove use in the facility.3 Anesthetics and drapes can mask the early signs of allergic reactions, and hypotension may be the first indication of distress. Rapid detection of an allergy to latex with immediate intervention is necessary to prevent serious complications and death from this severe immediate hypersensitivity reaction.4
Persons at Risk
It is extremely important to question all patients about the possibilities of latex allergy. The Food and Drug Administration has supported these recommendations since 1991.
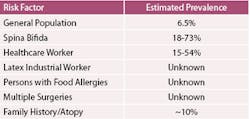
Certain populations are at higher risk for latex allergies than the general population. These risk factors should be discussed as a part of a thorough medical history to identify patients with these potential risk factors. (Table 3)
Spina Bifida
Children with spina bifida have a congenital disorder where a portion of the neural tube does not close. These children usually require multiple surgeries in infancy. The cause of natural rubber latex allergy in children with spina bifida is unknown, but some sources theorize the increased sensitization is a result of multiple latex exposure at an early age from multiple surgeries with mucosal contact.4,9,10
Health-care Workers
One of the first studies to determine latex glove allergy in healthcare workers occurred in Finland. Turjanmaa found a prevalence of 7.4% of operating unit physicians had immediate allergic responses to latex gloves,11 and described two case studies of a female surgeon and a female dentist exhibiting local allergic reactions following obstetric closure of episiotomy wounds. Dentists, dental hygienists and assistants have prolonged exposure to latex that is similar to operating staff because the nature of most dental procedures requires mucosal contact and therefore glove precautions. If powdered gloves are used, there is an increase in aerosolized particles of latex proteins each time gloves are removed.
Latex Workers
Although the rubber industrial worker has not been studied for latex allergy to the extent of the healthcare worker, several studies have indicated a higher prevalence in this population. The job type that was most frequently associated with a higher prevalence of latex allergy in the rubber industry was associated with tube curing and inspection. This was associated with higher dust levels, chemical curing fumes confined in small areas, and a synergistic effect between occupational exposure and cigarette smoking.3
Persons with Food Allergies
On the medical history questionnaire, allergies reported to fruits, milk, or nuts can be a strong indicator of potential problems with latex sensitivity.
Persons with History of Multiple Surgeries/Atopy
Patients with a history of multiple surgeries are at increased risk for latex sensitivity, especially if these surgeries were associated with the bladder or bowel. Patients with a familial history of hay fever, asthma, dry skin, or eczema are collectively termed atopy. Atopic individuals may be at an increased risk for the development of natural rubber latex allergies.
Diagnostic Tests for Latex Allergy
There are several tests used to identify and diagnose patients with allergies. A patient with a suspected latex allergy should be sent to an allergist or dermatologist for further evaluation. It is requested by the Centers for Disease Control and Prevention that all anaphylactic allergic reactions are reported.
RAST
Radioallergosorbent test (RAST) is an assay that measures immunogenicity to latex products. RAST has a low sensitivity (65%) and is not used as often as other testing methods discussed. The skin prick test is a reliable, cost effective, sensitive testing method that currently provides very reliable results for diagnosing natural rubber latex allergies. The skin prick test is an indicator of IgE sensitization and should be used cautiously in highly allergic patients because there have been anaphylactic reactions reported.3,12 One problem with this test is the lack of an FDA-approved standardized latex material used for the testing procedures. The skin prick test is performed by a trained allergist with resuscitation devices available to treat resulting anaphylactic episodes.
Patch Testing
Accelerators and antioxidants present in the gloves most often cause allergic contact dermatitis. The diagnostic procedure used to identify a type IV hypersensitivity reaction to latex is the patch test. Common contact allergens such as thiuram, phenylenediamine, and mercaptobenzothiazole are used in the patch test.2,3,11,12,13 This test will confirm an allergic contact dermatitis from an irritant contact dermatitis.
Use Test
The Use Test is performed on wet hands and is an assay to test patients who present with a negative serological history of natural rubber latex allergy, but have a history that suggests possible latex sensitivity. The Use Test begins with a 15-minute contact of a latex finger cot. The test is positive if urticaria or erythema is present. If there is no reaction, the entire glove is moistened with saline and placed on the hand for fifteen minutes. The test is negative if no reaction occurs as a result of the placement of either the glove or finger cot.
Cross-reactivity of Food Allergens
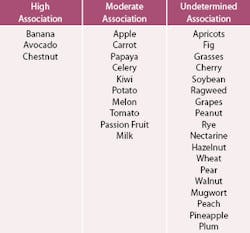
As mentioned previously, there are several foods that have been associated with cross-reactivity with latex hypersensitivity. Table 4 shows a sample of fruits, nuts, and allergens that have been shown to have similar epitopes or proteins that cross-react and should serve as a flag to the clinician as a potential natural rubber latex-sensitive patient.
Routes of Exposure
There are two routes of exposure to latex proteins associated with glove use. The first route is through direct contact with skin or mucosal surfaces. Water-soluble latex proteins can be absorbed through the skin, mucosa, and body fluids. Mucosal surfaces appear to absorb latex proteins much more readily than intact skin surfaces. Type IV hypersensitivity is more commonly associated with processing accelerators such as thiurams, mercaptobenzothiazoles, carbumates, and cow’s milk casein. Natural rubber latex protein exposure through direct contact is most often the cause associated with type I hypersensitivity. The second route of exposure to latex proteins occurs through inhalation. Corn-starched powdered gloves allow greater numbers of aerosolized latex protein particles to be suspended into the air. These particles can remain for over 12 hours. Each time gloves are removed, a greater number of suspended particles become aerosolized. By using non-powdered gloves, aerosolized latex proteins are significantly reduced.
Dental Products Containing Latex
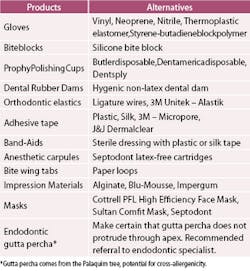
Table 5 contains some of the products used in dentistry that contain latex and a list of alternative products. It is difficult to determine latex content, so the manufacturer must be called to check whether or not products are latex-free.
Gloves
Gloves used in healthcare for standard precaution practices are the primary source of exposure to natural rubber latex in the healthcare worker and the patient. Surgical gloves are used for barriers during surgery. Examination (non-sterile) gloves are used for routine exams, restorative procedures, and preventive care. Thicker, less permeable, (utility) gloves are used during cleaning procedures. Each of these glove types can contain latex proteins in variable amounts, as well as processing chemicals that are responsible for type IV and type I allergic responses. It is important for the healthcare worker to remember these potential sources of exposure when a patient or they themselves are identified as having a latex hypersensitivity. “Hypoallergenic” was a label used on glove boxes prior to September 1998. This was a term used by glove manufacturers to indicate that the chemical accelerators and antioxidants responsible for contact dermatitis had been reduced. These gloves still contained latex, and the term was not an indicator of low-protein content. The term “hypoallergenic” has been determined by the Food and Drug Administration to be misleading and should no longer appear on glove box labels.
Summary
As a result of the increased use of barriers during medical and dental procedures, latex allergic reactions have also increased. Practitioners should take a thorough medical history and ask questions that could reveal a potential hypersensitivity to latex products. The healthcare worker is also at a higher risk than the general population of developing latex allergies. Several methods can be used to decrease exposure of the healthcare worker and the patient to latex. Knowing which products contain latex, and using alternative products, can reduce exposure to latex proteins. Low-protein non-powdered gloves can significantly reduce exposure to latex proteins in the office by reducing the amount of aerosolized latex proteins during donning and removal procedures. It is also possible to reduce delayed hypersensitivity reactions by using powder-free gloves or gloves that have been coated with a hydrogel.
Patients and healthcare workers who are latex-sensitive should wear Medic-Alert bracelets to identify their allergy because of the ubiquitous use of natural rubber latex products in medical settings. Patients who are latex-sensitive should be treated as the first patient of the day, and extreme care should be used to avoid cross-contamination of surfaces and the environment in general. Alternative products that are latex-free should be made available for their dental care needs. The cost of latex alternatives and nonlatex gloves has been analyzed and found to be less expensive when compared to disability and liability costs associated with latex products.1 Latex-safe environments should also be provided for the healthcare worker who is hypersensitive. Alternative products that do not contain latex and latex avoidance can significantly reduce the number of patients and healthcare workers who become latex-sensitive.
References
1. Phillips V, Goodrich M, TJ S. Healthcare Worker Disability Due to Latex Allergy and Asthma: A Cost Analysis. Am J Pub Health. 1999;89:1024-1028.
2. Melton A, Managing latex allergy in patients and healthcare workers. Cleveland Clin J Med. 1997;64:76-82.
3. Woods J, Lambert S, TAE P-M, Drake D, Edlich R. Natural Rubber Latex Allergy: Spectrum, Diagnostic Approach, and Therapy. J Emerg Med. 1996;15:71-85.
4. Spina A, Levine H. Latex allergy: A review for the dental professional. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:5-11.
5. Vlitalo L, Makinen-Kiljunen S, Turjanmaa K, Palosuo T, Reunala T. Cow’s milk casein, a hidden allergen in natural rubber latex gloves. J Aller Clin Immunol. 1999;104:177-180.
6. Fisher A, Contact urticaria and anaphylactoid reactions to cornstarch surgical glove powder. Contact Dermatitis. 1987;15:69-72.
7. Safadi G, Safadi T, Terezhalmy G, JS T, Battisto J, Melton A. Latex Hypersensitivity: Its prevalence among dental professionals. J Am Dent Assoc. 1996;127:83-88.
8. Harmann C, Turjanmaa K, Rieschel R, et al. Natural Rubber Latex Hypersensitivity: Incidence and Prevalence of Type I allergy in the Dental Professional. J Am Dent Assoc. 1998;129:43-54.
9. Shaer C, Meeropol E. Latex information: Spina Bifida Association of America. 1999.
10. Szepfalusi Z, Seidl R, Bernert G, Dietrich W, Spitzauer S, Urbanek R. Latex sensitivity in spina bifida appears disease-associated. J Pediat. 1999;134:344-348.
11. Turjanmaa K, Incidence of immediate allergy to latex gloves in hospital personnel. Contact Dermatitis. 1987;17:270-275.
12. Sussman G, Beezhold D. Allergy to Latex Rubber. Ann Int Med. 1995;122:43-46.
13. Berky Z, Luciano W, James W. Latex Glove Allergy: A survey of the US Army Dental Corps. J Am Med Assoc. 1992;268:3695-2697.
Disclaimer
This course has been made possible through an unrestricted educational grant from Hu-Friedy. The authors of this course have no commercial ties with the sponsors or the providers of the unrestricted educational grant for this course.
Reader Feedback
We encourage your comments on this or any ADTS course. For your convenience, an online feedback form is available at www.ineedce.com. Click here to view questionnaire