Risks of medication-related osteonecrosis of the jaw in cancer patients
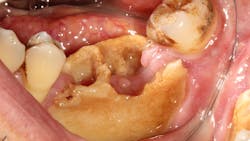
Medication-related osteonecrosis of the jaw (MRONJ) is a rare, yet severely debilitating, condition characterized by nonhealing exposed bone in individuals who have taken medications known as bone resorption inhibitors (BRIs) and antiangiogenic agents and have no history of radiation therapy of the jaw. Understanding who is at risk for developing MRONJ is vital to preventing this condition’s negative impact on quality of life and morbidity.
Medication-related osteonecrosis of the jaw symptoms and signs include:
- Pain, swelling, redness, or other signs of infection in the gum tissue
- Oral tissue that doesn’t heal after dental work
- Loose teeth
- Numbness or a heavy feeling in the jaw
- Drainage
- Exposed bone
Since treatment options for established MRONJ are limited, it is vital to identify patients who are at risk and prioritize preventive measures, ideally before administration of the first dose of medication.1 Recognizing at-risk patients includes knowledge of what makes one a candidate to receive BRIs and angiogenic inhibitors.
Bone resorption inhibitors
Our bones go through a remodeling process much like our teeth go through a constant routine of demineralization-remineralization. Bone remodeling is a coordinated effort between cells that build bone (osteoblasts) and cells that break down bone (osteoclasts). Bone resorption inhibitors (also known as bone-modifying agents or BMAs) interfere with this natural process by reducing the role and function of osteoclasts. Allowing bone to build without interruption increases bone density, which helps bones remain strong and intact.2
The first type of drug identified to cause osteonecrosis of the jaw (ONJ) was bisphosphonates (BPs), leading to the term bisphosphonate-related osteonecrosis of the jaw (BRONJ). BPs are a type of BRI that is approved for osteoporosis and also often used in cases involving osteopenia. They are also prescribed for less common diseases of the bone, such as osteogenesis imperfecta and Paget’s disease.
Pediatric cancer care and the dental professional
Effects of cancer treatment on periodontal condition and oral health
BPs interrupt the bone remodeling process by binding to the hydroxyapatite on the surface of bone. While 95% of a bisphosphonate will be released from the body within six hours, the half-life may last for more than 10 years. BPs can be administered either orally or via IV, and the risks of developing BRONJ is dose- and time-dependent. Names of BPs include, but are not limited to, zoledronic acid (Zometa, Reclast), alendronate (Fosomax), ibandronate (Boniva), and pamidronate (Aredia).
In 2014, the American Association of Oral and Maxillofacial Surgeons (AAOMS) released a position paper recommending that BRONJ be renamed MRONJ “to accommodate the growing number of osteonecrosis cases associated with other antiresorptive agents (denosumab) and antiangiogenic therapies.”3
Denosumab is another BRI that has a different mechanism of action compared to BPs. It disrupts the bone remodeling process, therefore helping to increase bone mineral density and reduce risks of fractures. Denosumab does not bind to bone, so it has a short half-life. The effects on bone are mostly gone within six months when the drug is discontinued. Brand names include XGEVA and Prolia.
Both BPs and denosumab are sometimes used in cancer care. Certain cancers and their treatments can leave patients at an increased risk for bone loss and conditions such as hypercalcemia, as well as skeletal-related events (SRE). Examples of SREs include bone pain, fractures, and spinal compressions.
Cancer-associated bone loss can be a result of multiple factors, including the direct effect of cancer cells and as a side effect of medications often used in cancer treatment such as corticosteroids, chemotherapeutic agents, aromatase inhibitors, and androgen deprivation therapies. Therefore, loss of bone mineral density is a greater concern for those being treated with hormone therapies for prostate cancer or hormone-receptor positive breast cancers.4
Most often we will see BRIs used for multiple myeloma and secondary cancers that have spread to the bone. The dosage for BRIs in cancer care tends to be much higher compared to that used for treating osteoporosis, which increases the risks of associated side effects. For example, the risks of denosumab-related osteonecrosis associated with osteoporosis therapy is 0.01%–0.03% versus 1%–2% with cancer patients.3
Angiogenic inhibitors
Angiogenic inhibitors prevent the process of blood vessel formation known as angiogenesis. Blood supply is needed to support solid tumor growth and the spread of cancer cells from one area to another, called metastasis. Inhibiting angiogenesis essentially starves the tumor to prevent or slow its growth.5 Being that osteonecrosis is classically considered an interruption in vascular supply, or avascular necrosis, it is logical that angiogenic inhibitors would be connected to the development of ONJ.1
ONJ has been reported with angiogenic inhibitors called tyrosine kinase inhibitors (TKIs) and monoclonal antibodies targeting VEGF (vascular endothelial growth factor). The US Food and Drug Administration has singled out specific medications within these classes—i.e., bevacizumab (Avastin) and sunitinib (Sutent). However, the AAOMS special committee on medication-related osteonecrosis of the jaw expressed concern for a similar potential risk associated with several medications within these same classes.3
When indicated, BRIs are used in conjunction with antiangiogenic medications. Patients receiving concomitant treatment with BRIs and VEGF-TKIs have a five to 10 times higher risk of developing MRONJ compared to patients treated with BRIs alone.6
What this means for the dental provider
The AAOMS supports a multidisciplinary approach that includes consultation, screening, and appropriate dental care before starting medications that pose a risk. A number of studies have demonstrated a significant reduction in the incidence of ONJ for patients who were provided these services prior to initiating drug therapy.3
Proactive treatment planning should include:1,3
- Thorough examination with radiographs
- Consideration of patient’s motivation when determining treatment plan
- Patient education about risks, signs, and osteonecrosis of the jaw symptoms
- Oral hygiene instructions to reduce the bacterial burden and prevent dental decay and infections while avoiding tissue trauma
- Lifestyle counseling on diet, tobacco, and alcohol
- Elimination of acute infections
- Completion of any remedial dental procedures
- Identification of potential future infection sources and extraction of teeth with poor prognoses
- Adjustment or replacement of ill-fitting dentures or partials to minimize tissue trauma
- Recommendation of personalized products, including prescription fluoride if indicated
- Use of antimicrobial rinses
If invasive dental care is necessary after medications have been administered (longer than four years with BPs alone or less than four years with BPs and concomitant use of an antiangiogenic), the prescribing physician should be contacted to discuss a drug holiday for at least two months prior to the procedure. Medications should not be continued (if the patient’s medical condition allows) until osseous healing and full mucosal coverage occurs.
Prevention needs to be the dental professional’s main focus since treatment options are limited. Continued collaboration between medical and dental professionals is essential to reduce the incidence of MRONJ. Early intervention, patient education, and preventive oral care can preserve the quality of life for patients at risk.
Editor's note: This content was originally published in 2021 and has been updated as of October 2025.
References
- Aldhalaan NA, Baqais A, Al-Omar A. Medication-related osteonecrosis of the jaw: a review. Cureus. 2020;12(2):e6944. doi:10.7759/cureus.6944
- How bisphosphonates work. Cancer Research UK. Updated November 27, 2019. https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/bisphosphonates/how-bisphosphonates-work
- Medication-related osteonecrosis of the jaw–2014 update. American Association of Oral and Maxillofacial Surgeons. https://www.aaoms.org/docs/govt_affairs/advocacy_white_papers/mronj_position_paper.pdf
- Drake MT. Osteoporosis and cancer. Curr Osteoporos Rep. 2013;11(3):163-170. doi:10.1007/s11914-013-0154-3
- Angiogenesis inhibitors. National Cancer Institute. Updated April 2, 2018. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet
- van Cann T, Loyson T, Verbiest A, et al. Incidence of medication-related osteonecrosis of the jaw in patients treated with both bone resorption inhibitors and vascular endothelial growth factor receptor tyrosine kinase inhibitors. Support Care Cancer. 2018;26(3):869-878. doi:10.1007/s00520-017-3903-5
About the Author

Jill Meyer-Lippert, RDH
JILL MEYER-LIPPERT, RDH, is the founder of Side Effect Support LLC, an online resource for cancer survivors and health-care providers to reduce harmful oral side effects of treatments. She also provides communications and educational engagement services as the community relations manager for Custom Dental Solutions. Meyer-Lippert received the 2014 Sunstar/RDH Award of Distinction and is a member of the Triage Cancer Speakers Bureau and the RDH Advisory Board for the Oral Cancer Foundation.