It's ‘refractory'
What exactly does that mean?
by Larry Burnett, DDS
I recently received a nice note from Dr. J. Fernandez asking my opinion concerning the theory that bacteria associated with periodontal disease can penetrate soft tissue and thereby escape treatment.
“I've found studies that seem to show this. Then I find others that suggest this phenomenon hasn't been confirmed. What are your thoughts?”
Great question!
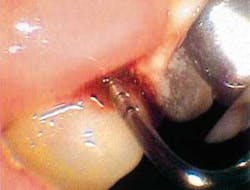
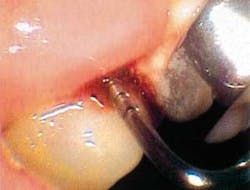
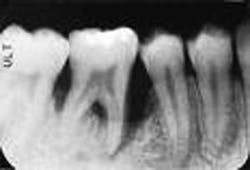
But before we discuss soft tissue invasion, I'd like to talk a little about refractory periodontitis. We've all had cases where the patient fails to respond to treatment. We do everything we can think of to stop the attachment loss, yet at each recall, the probe slides a little farther into the pockets and the radiographs look bleaker (see Figures 1 and 2).
Some treat the term “refractory periodontitis” as if it were a type of disease. Others use it to mean “untreatable.” In fact, “refractory” simply refers to any stubborn disease that has not responded to conventional treatment. In this little article I'd like to suggest that calling a periodontal condition “refractory” simply means we haven't yet reached the pathogens that are triggering the tissue loss.
Take juvenile periodontitis, for example. Until the 1980s, juvenile periodontitis was a mysterious, untreatable disease that defied scaling, root planing, and periodontal surgery. Despite all of the recognized therapies, the disease seemed destined to run its course.
It was the quintessential “refractory” periodontal disease.
This suddenly changed when researchers discovered antigens to a single bacillus, Actinobacillus actinomycetemcomitans (A.a), in the periodontal tissue of more than 90% of juvenile periodontitis sufferers. This antigen was in virtually 0% of the tissue of healthy patients or patients suffering conventional periodontitis.
It's rare that a single periodontal disease is so closely identified with a single pathogen. But it gets even more interesting ...
Occasionally, antigens to A.a would be discovered in a healthy patient. But when this occurred, it was always in the plaque — never in the tissue itself.1 So apparently juvenile periodontitis wasn't just associated with a specific pathogen (A.a) but with a specific pathogen in a specific location.
It's a shame all studies are not as definitive as these, because this work leads to one of the few true “Eureka!” moments in dental science. The findings suggested a new model for juvenile periodontitis, one that clearly explained why it had been so resistant to conventional therapy. Other studies suggested that A.a may actually have developed the ability to suppress the body's immune system, preventing the synthesis of antibodies.2
Once pathogens penetrate beyond the pocket into the connective tissue, they are not susceptible to mechanical debridement or root planing. The tissue would even protect them against antibacterial irrigants that would be lethal against free-swimming beasts. (Not that many were irrigating back then.) Since they had evolved to an ability to suppress antigens, the body's natural defense against pathogens in tissue was less effective.
It was a classic example of the “locus of infection.” Even if you managed to get a pocket clean, the hidden beasts in the tissue would immediately emerge to repopulate the crevicular area.
This model also suggested a potential treatment. Instead of attacking the A.a from the outside with curettes, what if we attacked them from the inside? At that time, systemic antibiotics were seldom used for treating periodontitis. But tetracycline was found to be effective. Later, Dr. Loesche et al. showed that amoxicillin combined with metronidazole is even more effective in treating juvenile periodontitis.
Suddenly, most cases of “refractory” juvenile periodontitis were no longer refractory.
So in answer to your question, Dr. Fernandez, absolutely yes! Periodontal bacteria can penetrate the soft tissue. That's now the generally-accepted model for juvenile periodontitis.
And it's probably not just A.a either. Treponema denticola is an anaerobic spirochaete that in conjunction with Treponema socranskii and Porphyromonas gingivalis, is associated with a particularly aggressive periodontitis.
Sudies suggest that T.d emits a protein-destroying chemical that attacks the tight junctions between epithelial cells to allow penetration.3 P. gingivalis has also been described as a tissue-invasive pathogen.4
Though most researchers agree that certain periodontal pathogens can penetrate the soft tissue, how frequently this happens in cases other than juvenile periodontitis is open to debate.
Personally, I suspect that the model of the “hidden pathogen” explains much (maybe even most) “refractory” periodontal disease. Critics of antimicrobials point out that they also eliminate the “good” microbes that compete with the “bad” ones. In typical cases of periodontal disease, I'm not much concerned about that, because the benign forms quickly repopulate the pocket. However, what if the “hidden pathogens” escaped local treatment? Since the therapy has killed all the normal residents of the pocket (both good and bad), the offending pathogens will have no competition when they re-emerge. So they'll easily repopulate the pocket.
The beasts don't have to be hiding in tissue, of course. They may be hunkered in grooves, furcations, the bottom of tortuous pockets or inside vital or nonvital teeth. Even if they don't penetrate, they may attach to the soft tissue wall and be partially submerged in ulcerated tissue lining the pocket.
But I personally believe that tissue-penetration may explain a large percentage of “refractory” cases.
In what I consider to be a very important study, researchers at Boston's Forsyth Institute identified 14 patients as “refractory.” These patients continued to lose attachment despite traditional comprehensive treatment consisting of scaling and root planing, surgery, plus systemic antibiotics.
These desperate patients were then treated using:
- Scaling and root planing
- Two weeks of systemic amoxicillin plus metronidazole (or alternatively locally delivered tetracycline)
- Weekly professional supragingival plaque removal for three months
At the end of the three-month active program, 100% of the patients showed a major reduction in pernicious periodontal pathogens. And despite their “refractory” classification at the beginning of the study, when the two-year monitoring period ended, all patients were better off than when they started. Most still exhibited a drop in microflora; the average attachment gain was .44mm — the average pocket reduction was .83mm.5
Notice that the effective treatment for these refractory cases was nothing revolutionary. Rather, it was a ratcheting up of the previous efforts. It consisted primarily of using a more lethal antibiotic regimen and more rigorous debridement.
One subject that has fascinated me for almost a decade is the potential of “sonoporation” to treat recalcitrant cases of periodontal disease.
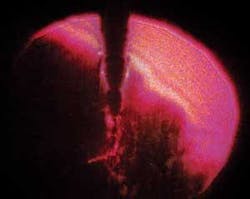
Sonoporation refers to transdermal drug delivery using ultrasonics. When something vibrates ultrasonically in a liquid, the pressure oscillations created can raise the temperature of small regions of the liquid to the boiling point. This creates a stream of microscopic cavitation bubbles flowing out from the vibrating source. When the bubbles collapse, a jet of fluid rushes through the center of the bubble with incredible pressure (see Figure 3). You could think of this jet as a microscopic battering ram or as an injection.
In sonoporation, collapsing cavitation bubbles are used to loosen epithelial cells, making the skin barrier permeable enough for large molecules to penetrate. Obviously, if the pathogens are hiding from traditional therapy in a soft-tissue hideout — and if ultrasonic delivery can force antimicrobials into their lair — ultrasonic delivery of medicaments should help resolve “refractory” periodontitis.
Dental ultrasonic tips have been designed to remove calculus or plaque. I don't know of any tips designed specifically to optimize the amount of cavitation they create. Several years ago I worked with Tom Matulo, PhD, of the University of Washington's Applied Physics Laboratory, exploring the possibility of getting government funding to examine ultrasonic design specifically to enhance the ability to deliver pathogen-destroying medicaments in a pocket.
We didn't get the grant; so, ultimately, we had to drop the project. But Tom's preliminary research was fascinating. He showed that the cavitation did indeed occur around an ultrasonic tip, but that its pattern varied dramatically depending on tip design, oscillation frequency, and power setting (see Figure 4). This suggested that the effectiveness might be improved significantly to optimize delivery of medicaments.
This is all largely speculative. But it might explain why the research concerning ultrasonic application of antimicrobials remains so mixed. The current consensus appears to be that for patients suffering mild-to-moderate periodontitis, the difference between water and antimicrobial in the ultrasonic lavage is not clinically significant. Both types of patients seem to get better. However, for patients with severe pockets, particularly with complicating factors such as insulin-dependent diabetes or those who are immunocompromised, adding chlorhexidine or povidone iodine to the lavage may improve the prognosis.
Perhaps (and remember, this is speculation) in severe cases, the pathogens have penetrated the tissue, and sonoporation forces the antimicrobial agent into the tissue where the beasts are hiding. So in these cases, the marriage of ultrasonics and antimicrobials may make the difference.
References
- Christersson LA, et al. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. Light immunofluorescence and electron microscope studies. Jour Periodontol. 58(8), p529-39, 87.
- Kurita-Ochiai T, et al. Adoptive transfer of suppressor T-cells induced by Actinobacillus actinomycetemcomitans regulates immune response. J Periodontl Res. 29:1-8, 1994.
- Chi B, et al. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res. Microbilo. 154:9, p637-43, Nov 03.
- Holt SC, et al. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 2:177-281, 1991.
- Haffajee AD, et al. Clinical and microbiological changes associated with the use of combined antimicrobial therapies to treat “refractory” periodontitis. Jour of Clin Perio, Vol 31:10, p. 869-877, Oct 2004.