Lichen planus
Patients are asking if amalgam removal would help
Nancy W. Burkhart, BSDH, EDD
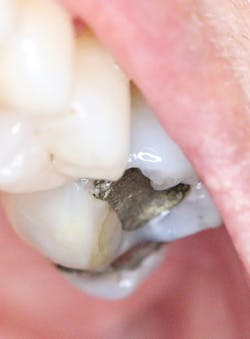
Amalgam on #14. Courtesy of Carol Perkins, RDH, BA.
Your patient today is Jennifer, a patient of record who has been diagnosed with oral lichen planus confirmed through a biopsy. During your initial assessment, Jennifer asks the following question: “If I have oral lichen planus, should I remove my amalgams because of mercury leakage? I have seen postings on the internet and engaged in conversations on this subject suggesting that amalgam/mercury may be contributing to my oral lesions.”
This question is asked frequently by patients who have obtained information from various sources and are concerned about the long-term effects of mercury in the environment, particularly in their own mouths. Many patients want to know if removing amalgams will assist in improvements with oral lichen planus or their general health. Since lichen planus normally affects the adult population (mainly in age groups over 40 years of age), many patients with lichen planus also may have one or more amalgam restorations. Amalgam was the restoration of choice for many years in the United States. Many offices have stopped using amalgam mainly because better restoration materials have come on the market in the past several decades. With this said, many clinicians will tell you that amalgam is a strong material and, when properly placed, will last for decades.
The lasting quality of amalgam is not a debate. However, when amalgam leaks or when the dental material is removed, mercury vapor is released in the form of elemental mercury, and is absorbed by the lungs and the body (FDA, 2015). High levels affect the lungs, kidneys, and brain. Mercury from dental amalgam and other sources (fish, for example) is bioaccumulative.
Mercury is also released in extremely small amounts while normal functions are performed such as chewing, eating, brushing, grinding of teeth and even polishing teeth.
According to the FDA: “Some individuals have an allergy or sensitivity to mercury or the other components of dental amalgam (such as silver, copper, or tin). Dental amalgam might cause these individuals to develop oral lesions or other contact reactions. If you are allergic to any of the metals in dental amalgam, you should not get amalgam fillings. You can discuss other treatment options with your dentist.”
Positions from the ADA (2016) state that there is no association with Alzheimer’s disease, Parkinson’s disease, lupus, multiple sclerosis, and other degenerative diseases that have been suggested in past articles. The statement cites multiple studies from reputable journals.
Answering the question
Some patients are asking if all amalgams should be replaced and if the mercury leakage that may occur in older restorations is contributing to their oral lichen planus. Most of the time, oral tissue that is in direct contact with an amalgam restoration or perhaps a metal (gold) crown may exhibit what is called a “lichenoid” reaction.
A dermatologist can perform what is termed “patch testing” and other tests to determine if there is evidence of metal sensitivity. Reports of amalgam removal that is linked to tissue reaction has been known for many years. Sometimes the tissue contacting the metals will improve upon removal of the amalgam or gold crown.
Some patients with oral lichen planus appear to be removing their amalgams, with the hope of improving their oral lichen planus or overall health.
Even when biopsied and viewed under a microscope, the diagnosis is often unclear and may appear as lichen planus. Some pathologists will diagnose this as “lichenoid mucositis” or others may indicate just “a lichenoid reaction.” Documenting before and after images will usually answer this question. Again, a dermatologist can usually determine if metal sensitivity may be an issue.
There have been posts on the internet recently suggesting that patients with lichen planus have all their amalgams removed. This article is not suggesting that everyone begin removing amalgam when possible. If a restoration needs to be replaced, dentists will most likely use a newer material to replace an amalgam.
Results from a five-year follow-up of patients who had high mercury concentration in their urine before amalgam removal reported improved health conditions. The study was considered subjective and based on patient reported data in a small study population.
Other studies report an improvement too and believe there is a systemic allergenic/irritant potential (Sharma, et al. 2015). The FDA (2015) suggests that the developing neurological systems in fetuses and young children may be more sensitive to the neurotoxic effects of mercury vapor. Therefore, pregnant women and young children may be more at risk. This of course, would especially be a concern for dental professionals who work around mercury vapors in the dental office and especially those who may be pregnant.
The FDA and EPA has suggested that consuming fish (methylmercury) with potentially higher mercury levels should be avoided. Fish such as tilefish from the Gulf of Mexico, shark, swordfish, orange roughy, bigeye tuna, marlin, and king mackerel are known to have higher levels of mercury. Lower levels of mercury are found in seafood such as shrimp, pollock, salmon, canned light tuna, tilapia, catfish, and cod. As stated above, the FDA asserts that mercury from dental amalgam and other sources (fish) is bioaccumulative in organs. Therefore, limiting the exposure to added mercury sources is optimal.
Again, the intent is not to promote amalgam removal, but since some patients with oral lichen planus appear to be removing their amalgams, with the hope of improving their oral lichen planus or overall health, some safety tips are warranted.
The following organizations appear to be cohesive with their recommendations:
- The Food and Drug Administration does not suggest amalgam removal when the restorations are not cracked, leaking, or there is no evident decay under the amalgam.
- The American Dental Association stated in 2009 that the “scientific evidence supports the position that amalgam is a valuable, viable, and safe choice for dental patients.”
- The Canadian Dental Association states, “It is considered unnecessary and ill-advised to replace functional or serviceable dental amalgam fillings (restorations) for safety concerns or perceived health needs. A conservative approach to filling replacement, combined with effective decay prevention, is strongly advised to help maintain the dentition over a lifetime.”
- The International Academy of Oral Medicine and Toxicology has specific guidelines established in December 2016 for the removal of amalgam restorations. These guidelines can be found at thesmartchoice.com.
Images of the areas in which visible lichen planus is evident may be documented through digital images and may be assessed later to determine if improvement of a possible lichenoid reaction may have occurred.
Replacement is only recommended when there is breakage, leakage, and decay involved. Patients who are informed about the amalgam removal process will certainly have a better outcome and be able to plan for their needs at the time. A dermatologist will be able to determine if metal sensitivity may be a factor in patients with oral lichen planus. Continue to ask good questions, and always listen to your patients.
NANCY W. BURKHART, BSDH, EdD, is an adjunct associate professor in the department of periodontics-stomatology at the Texas A&M College of Dentistry in Dallas. Dr. Burkhart is founder and cohost of the International Oral Lichen Planus Support Group (dentistry.tamhsc.edu/olp/ and coauthor of General and Oral Pathology for the Dental Hygienist, which is in its third edition. She was awarded a 2016 American Academy of Oral Medicine Affiliate Fellowship (AAOMAF). She was a 2006 Crest/ADHA award winner, a 2012 Mentor of Distinction through Philips Oral Healthcare and PennWell Corp, a 2017 Dental Professional of the Year through The International Pemphigus and Pemphigoid Foundation, and a 2017 Sunstar/RDH Award of Distinction Recipient. She can be contacted at [email protected].
References
1. ADA Statement on Dental Amalgam. http://www.ada.org/en/about-the-ada/ada-positions-policies-and-statements/statement-on-dental-amalgam
2. ADA: http://www.ada.org/en/press-room/press-kits/dental-fillings-press-kit/dental-amalgam-what-others-say
3. ADA Council on Scientific Affairs: Amalgam Safety Update 2010. ADA.org. Accessed 12-6-2017.
4. Al‐Hashimi I, Schifter M, Lockhart B, Wray D, Brennan M, Migliorati CA, et al. Oral lichen planus and oral lichenoid lesions: Diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103(Suppl): S25.e1‐12.
5. Bjorkman L, Sjursen TT, Dalen K, et al. Long term changes in health complaints after removal of amalgam restorations. ACTA ODONTOLOGICA SCANDINAVICA, 2017 VOL. 75, NO. 3, 208–219.
6. Canadian Dental Association Position Paper on Amalgam: https://www.cda-adc.ca/_files/position_statements/amalgam.pdf Accessed 12-5-17.
7. Sharma R, Handa S, De D, Radotra BD, Rattan V. Role of dental restoration materials in oral mucosal lichenoid lesions. Indian J Dermatol Venereol Leprol 2015;81:478‐84.
8. FDA “Information for Use.” FDA and EPA issue final fish consumption advice [press release]:The US Food & Drug Administration; Published January 18, 2017.
9. White Paper: FDA Update/Review of potential adverse health risks associated with exposure e to mercury in dental amalgam. National Center for Toxicological Research, U.S. Food and Drug Administration. FDA White Paper (2006) and Addendum (2009):
10. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DentalProducts/DentalAmalgam/ucm171117.htm