Stannous fluoride dentifrices: Providing protection for a lifetime
Since their introduction in the 1950s, fluoride compounds have significantly improved the dental health of our population.
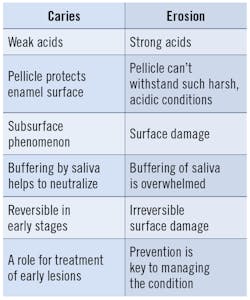
Fluoride dentifrice formulations have the ability to improve hard-tissue (enamel) densities. Before teeth decay, erode, chip, or crack, they usually undergo density loss. During this process, calcium is lost and the hydroxyapatite crystals are weakened. Clinically, changes manifest as dental caries and erosive tooth wear. However, while acid is the common etiologic factor, the modes of action are different and have different clinical outcomes (figure 1).
Dental caries
Dental caries is the localized destruction of susceptible dental hard tissues by acidic by-products from the bacterial fermentation of carbohydrates.1 The prevalence of dental caries, often referred to as decayed, missing, or filled teeth (DMFT), has leveled off or even decreased in recent years,2 but remains a major clinical problem, especially among underserved communities.3 The caries process is well understood and involves several other processes. Tooth enamel is highly mineralized and thus subject to demineralization from oral acids. With caries, the source of the acid is acidogenic bacteria that reside in protected areas (e.g., grooves, interproximal areas) within the acidic dental plaque. As carbohydrates are consumed, these compounds are ingested by bacteria and metabolized as acids, which decreases the acidity of the dental plaque. Also, in a low-acid environment, healthy bacteria are converted to acid-forming bacteria, further exacerbating the acidity of the plaque.4 This, in turn, leads to demineralization of enamel and cavitation into dentin tooth structure.
Prevention or interruption of this cycle of events occurs, most importantly, by the removal of plaque with brushing or flossing.
Diet modification and restriction of cariogenic foods are also important. Another key tactic is to interrupt the caries process with various dentifrices. Historically, sodium fluoride toothpaste formulations have been beneficial in helping to maintain mineral density of enamel and thus in reducing the rate of caries. However, sodium fluoride benefits are limited, and protective benefits can be improved by using a
Regarding prevention of caries, stabilized stannous fluoride dentifrices provide both bacteriostatic and bactericidal benefits (i.e., the ability to both inhibit and kill bacteria).6 Compared to sodium fluorides, stannous fluorides have shown a reduction of caries of 25.5%.5
Further improvement in caries control can be achieved by following the well-established Caries Management by Risk Assessment(CAMBRA) guidelines.7 This assessment model emphasizes that it is the oral environment that determines how biofilms behave and whether disease is severe enough to trigger changes in hard-tissue structures. Using stannous fluoride will provide additional benefits toward the prevention of caries.8
Erosive tooth wear
Erosive tooth wear involves two distinct processes. Teeth are first
Related reading:
- Using fluoride varnish to combat pandemic-related oral health risks
- Tipping the balance toward remineralization
- The efficacy and benefits of fluoride varnish with CPP-ACP
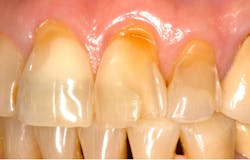
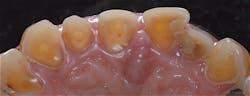
The prevalence of erosive tooth wear can vary during different periods of life (figure 4). A recent study noted that nearly 100% of children between the ages of 11 and 14 showed signs of erosive tooth wear.11 It should be noted again that unlike caries, which can be reversed with remineralization, erosive tooth wear is an irreversible process that continues throughout one’s lifetime. Minerals are removed directly from the surface, leaving a still-intact demineralized layer that is easily worn away.
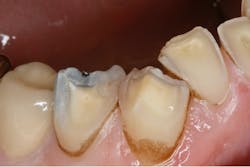
Bruxism has historically been considered the dominant factor in tooth wear. However, this may not be true, as studies have shown that under normal functional loads, in the absence of acid softening, there is very little wear from grinding.12 For example, as shown in figure 5, patients with severe acid challenges (e.g., GERD, xerostomia) can exhibit extensive tooth structure loss, even where surfaces have no direct occlusal contacts. It is therefore imperative during examinations and health history reviews to inquire about dietary habits, digestive and sleeping problems, and even daytime habits such as nail- biting and clenching. Dental hygienists are in an excellent position to obtain this information and provide patient guidance.
It should be noted that in relation to wear and tooth structure loss, it is especially important to recommend the use of a stannous fluoridetoothpaste. Only stannous fluoride can provide a protective coating to help minimize tooth damage from acid and mechanical wear.13
Preventive measures
Patient education is key when it comes to preventing dental problems. It is important for patients to understand that it’s not just about what they drink, but rather how they consume acidic, sugar-sweetened beverages (figure 6). Drinks should be swallowed, not sipped or swished in the mouth. Erosive tooth wear is, in large part, a function of acid coming in contact with the teeth, and this contact time should be minimized.14 It should be noted that when using a stannous fluoride dentifrice, the salivary (acquired) pellicle, which covers and protects tooth surfaces, is also saturated by stannous fluoride compounds. This provides an additional layer of protection against acids.15
Use of a stannous fluoride dentifrice is very impactful. As noted previously, a mostly insoluble layer of stannous (tin) is laid down on the tooth
surface, which acts as a protective physical barrier.13 Sodium fluoride does not provide this protection.16 Figure 7 shows a summary of the oral health benefits of using a stabilized stannous fluoride toothpaste.
Conclusion
Oral acids from various sources result in hard-tissue loss on teeth. Therefore, dental caries and erosive tooth wear can be regarded as “acid diseases.” These changes continue to occur throughout life as various etiologic factors transition with age.
Stabilized stannous fluoride dentifrices have been shown to protect against acid challenges while providing additional oral-health benefits. The use of a stabilized stannous fluoride toothpaste has the proven potential to help improve the oral health of patients over their entire lifetimes.
Editor's note: This article appeared in the November 2021 print edition of RDH and has been updated as of December 2025.
References
- Longbottom C, Huysmans MC, Pitts N. Glossary of key terms. Monogr Oral Sci. 2009;21:209-216.
- Fontana M, Young DA, Wolff MS, Pitts NB, Longbottom C. Defining dental caries for 2010 and beyond. Dent Clin North Am. 2010;54(3):423-440. doi:10.1016/j.cden.2010.03.007
- Chaffee BW, Rodrigues PH, Kramer PF, Vítolo MR, Feldens CA. Oral health-related quality-of-life scores differ by socioeconomic status and caries experience. Community Dent Oral Epidemiol. 2017;45(3):216-224. doi:10.1111/cdoe.12279
- Marsh PD. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am. 2010;54(3):441-454. doi:10.1016/j.cden.2010.03.002
- Stookey GK, Mau MS, Isaacs RL, Gonzalez-Gierbolini C, Bartizek RD, Biesbrock AR. The relative anticaries effectiveness of three fluoride-containing dentifrices in Puerto Rico. Caries Res. 2004;38(6):542-550. doi:10.1159/000080584
- Wefel JS, Stanford CM, Ament DK, et al. In situ evaluation of sodium hexametaphosphate-containing dentifrices. Caries Res. 2002;36(2):122-128. doi:10.1159/000057870
- Jenson L, Budenz AW, Featherstone JD, Ramos-Gomez FJ, Spolsky VW, Young DA. Clinical protocols for caries management by risk assessment. J Calif Dent Assoc. 2007;35(10):714-723.
- Ramji N, Baig A, He T, et al. Sustained antibacterial actions of a new stabilized stannous fluoride dentifrice containing sodium hexametaphosphate. Compend Contin Educ Dent. 2005;26(9 Suppl 1):19-28.
- Lussi A, Carvalho TS. Erosive tooth wear: a multifactorial condition of growing concern and increasing knowledge. Monogr Oral Sci. 2014;25:1-15. doi:10.1159/000360380
- Shellis RP, Addy M. The interactions between attrition, abrasion and erosion in tooth wear. Monogr Oral Sci. 2014;25:32-45. doi:10.1159/000359936
- Salas MM, Nascimento GG, Vargas-Ferreira F, Tarquinio SB, Huysmans MC, Demarco FF. Diet influenced tooth erosion prevalence in children and adolescents: results of a meta-analysis and meta-regression. J Dent. 2015;43(8):865-875. doi:10.1016/j.jdent.2015.05.012
- Zheng J, Huang H. Shi MY, Zheng L, Qian LM, Zhou ZR. In vitro study on the wear behaviour of human tooth enamel in citric acid solution. Wear. 2011;271(9-10):2313-2321.
- Faller RV, Eversole SL. Protective effects of SnF2 - part III. Mechanism of barrier layer attachment. Int Dent J. 2014;64(Suppl 1):16-21. doi:10.1111/idj.12098
- St. John S, Suszcynsky-Meister E, Schneiderman S. Acid exposure dominates erosive tooth wear etiology independent of toothpaste abrasiveness in laboratory method correlated to in situ results. Oral Health and Prev Dent. Submitted 2021.
- Hara AT, Zero DT. The potential of saliva in protecting against dental erosion. Monogr Oral Sci. 2014;25:197-205. doi:10.1159/000360372
- West NX, He T, Zou Y, DiGennaro J, Biesbrock A, Davies M. Bioavailable gluconate chelated stannous fluoride toothpaste meta-analyses: effects on dentine hypersensitivity and enamel erosion. J Dent. 2021;105:103566. doi:10.1016/j.jdent.2020.103566
About the Author

Warden H. Noble, DDS, MS, MSEd
Warden H. Noble, DDS, MS, MSEd, graduated from the University of California, San Francisco, and then completed a specialty program in prosthodontics at the University of Michigan. After 35 years in private practice, he accepted a position as professor in the Department of Integrated Restorative Sciences at the University of Pacific. He has given over 250 presentations both nationally and internationally, and has written numerous articles and chapters for dental texts.
Updated October 4, 2021