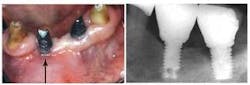
Controlling the Intraoral Environment Before and After Implant Therapy
by Richard Nejat, DDS, Daniel Nejat, DDS, Fiona M. Collins, BDS, MBA, MA
Educational Objectives
Upon completion of this course, the clinician will be able to do the following:
- Understand the process of patient selection and the systemic considerations that affect candidacy for implant treatment
- List the adverse implant outcomes due to biological/microbiological factors and mechanical factors
- Control the intraoral environment during all three phases of implant treatment-presurgical, postsurgical, and maintenance
- Understand the precautions to be taken when using instruments around implants and the potential damage that can occur
Abstract
Dental implants are a well-accepted treatment for the replacement of missing teeth. An estimated two million implants are placed annually, and it can be anticipated that an increasing number of implants will be placed and need to be maintained in the coming years. The intraoral environment and overall health of an individual patient influence patient selection/implant candidacy and the outcome of implant therapy. Factors affecting the intraoral environment include the patient’s heath status, medication use, level of oral hygiene, and habits such as smoking and drinking. Oral hygiene is an important determinant of implant success, as it is with the health of the natural dentition. Brushing and flossing are also critical success factors, requiring considerable patient education and motivation, and adjunctive therapy may be considered. With careful patient selection and patient commitment to oral hygiene measures, the potential for long-term success and implant health is excellent.
Introduction
Over the last two decades, oral implants have become a mainstream treatment for the replacement of missing teeth. It has been estimated that approximately two million implants are placed annually.1 Given the generally good success rates of oral implants, patient knowledge and interest in this treatment, as well as population demographics and dental trends, it can be anticipated that an increasing number of implants will be placed and need to be maintained in the coming years.
The intraoral environment and overall health of an individual patient influence patient selection/implant candidacy and the outcome of implant therapy. In periodontal health, plaque levels are low and relatively few periodontal pathogens are present. As plaque matures, the quantity of gram-negative organisms increases. As the number of periodontal pathogens increases, gingivitis and subsequently periodontitis may ensue, depending upon the host response and its severity.
Factors affecting the intraoral environment include the patient’s health status, medication use, level of oral hygiene, frequency of maintenance recall visits, and habits such as smoking and drinking. The association between systemic disease and periodontal health is well established, and the relationship between periodontal health and peri-implant health is well established. For short- and long-term success of implants, patients must be willing and able to perform effective oral hygiene measures to control the intraoral microbial environment.
Patient Selection
Patient selection during implant treatment planning involves many considerations. In addition to the intraoral environment, the patient’s general health status and smoking habits are highly relevant.
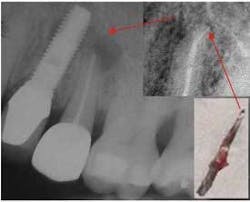
Peri-apical implantitis caused by a fractured endodontic file resulting in a peri-apical infection on the adjacent implant
Systemic Considerations
Diabetics are at increased risk for inflammatory periodontal disease, and there is a strong correlation between untreated periodontal disease and poor glycemic control.2 There have been several studies on the success rates of implants in diabetic patients. In one, implant failure rate was found to be slightly higher for diabetics than for nondiabetics, and failure occurred during the first year.3 A second study found significantly more failures in type 2 diabetics, but when implants were compared among patients the differences were found to be minimal.4 Overall, studies show implants to be successful in diabetics, and it has been concluded that they are not contraindicated unless the diabetes is uncontrolled.5
Medications affecting oral health and implant candidacy include anticoagulants, steroids, immunosuppressants, bisphosphonates, and drugs associated with gingival hyperplasia, such as calcium blockers and phenytoin. Patients on anticoagulants are at risk for hemorrhage, while patients on steroids and immunosuppressants are at risk for impaired healing and infection. Oral hygiene is physically impeded in patients with gingival hyperplasia and may result in a compromised intraoral environment with an increased presence of periodontal pathogens.
Chemotherapy patients also require special consideration. Implant placement in a patient receiving chemotherapy has been found to be associated with a high rate of failure and is contraindicated until blood profiles are back to normal.6 Bisphosphonates are not metabolized and have a strong binding affinity with osteoclasts. The mechanism underlying the reaction is unknown, but it has been postulated that bisphosphonates inhibit new vessel formation, thereby impairing healing. Patients on bisphosphonates are also at risk for osteonecrosis. When osteonecrosis (painful exposed bone) is present, effective control can be obtained using a regimen of antibiotics along with 0.12 percent chlorhexidine mouthrinse.7 Because this condition and its complications result in significant chronic pain, dysfunction and disfigurement which are difficult to treat, the focus should be on prevention. It is important that all health professionals, especially dentists and oncologists, be aware of this possibility with patients being considered for dental extractions or any surgical dental procedure and who are undergoing intravenous bisphosphonate therapy. It is also important for patients to be informed of the risk of this complication of bisphosphonate therapy, so that they have the opportunity to assess the need for dental treatment before starting therapy.8 Presently, it is not known whether discontinuing bisphosphonates before major dental procedures can help prevent the problem, but, given the persistence of bisphosphonates in bone, it is unlikely. Dentists and physicians should also be aware that osteonecrosis of the jaw can occur in association with oral bisphosphonate therapy for osteoporosis.9
Head and neck radiation has a number of deleterious sequelae. With respect to implant therapy, the risk of osteoradionecrosis is notable, can result from interventions as routine as scaling and root planing,10 and is life-threatening. While hyperbaric oxygen prior to surgery has been shown to help prevent osteoradionecrosis,11 implant surgery is nonetheless an intervention that places these patients at risk. Very careful consideration of the risks versus potential benefits is required. Where implants have been placed in irradiated bone, the failure rate can be up to 30 percent.12 Oral microbial shifts occur in head and neck radiation patients; however these are related to cariogenic bacteria and do not appear to be related to periodontal pathogenic bacteria. Recent research has found no shift in periodontal pathogens, suggesting no increased risk of periodontal disease or peri-implantitis.13
Smoking has consequences for systemic health, oral health, and implant success. Just as smoking is a risk factor in the progression of periodontal disease and is believed to increase the risk of periodontitis by at least 300 percent,14,15 it is also a risk factor for implant failure. Clinical research and retrospective studies have demonstrated a reduced implant success rate in smokers16 -a failure rate of 11.3 percent has been reported versus 4.8 percent in nonsmokers.17 Smokers have higher bleeding indices, visibly more inflammation, deeper depths upon probing, and more bone loss around implants, as evidenced on radiographs.18 Research has found that the amount of bone loss is correlated to the number of cigarettes smoked by individual patients.19
Drinking more than 10g of alcohol daily also appears from recent studies to be correlated with peri-implant bone loss.20
The age of a patient is often regarded as a consideration.21 Age, per se, is not an issue, but with an increase in age the patient may have systemic health or medication issues that either preclude treatment or affect the outcome. An increase in age has not been found to be directly correlated with either osseointegration or crestal bone resorption.22 Older patients may be less able to withstand surgery in general and have more difficulty maintaining the scrupulous oral hygiene required postsurgically and during the maintenance phase.
Patient selection must fully consider systemic health and all medication use, together with consultation with the patient’s physician.
Patient Selection: Intraoral Microbial Considerations
Important intraoral considerations include the patient’s oral hygiene and periodontal status (as well as the amount and quality of bone present at the implant site).23
Oral Hygiene is an important factor in implant success. The oral flora associated with healthy gingivae have been found to be the same as that found around healthy implant sites. Conversely there are similarities between the microbial composition associated with gingivitis and peri-mucositis and that associated with periodontitis and peri-implantitis.24 Patients with active periodontal disease have an increased risk for peri-implantitis25 and implant failure, which is noteworthy given the correlation between patients’ periodontal disease experience and implant failures. Approximately 30 to 35 percent of tooth extractions are due to periodontitis in patients over the age of 40.26 It has also been shown that periodontal pathogens migrate or “translocate” to implant sites and that reducing microbial loads prior to surgeries improves healing and success rates. Periodontal pockets act as reservoirs for pathogenic bacteria27 and should be treated prior to implant surgery.
Peri-apical lesions adjacent to implant sites can result in the lesions increasing in size following implant placement and, ultimately, implant failure.28 Retrograde peri-implantitis can occur due to lesions at an adjacent tooth or lesions associated with the site of a root extracted as an endodontic failure.29 Screening and successful treatment of peri-apical lesions as well as retained roots and foreign bodies should therefore precede implant treatment.
Microbes, Adverse Outcomes, and Treatment
Adverse implant outcomes include those due to biological/microbiological factors as well as those caused by mechanical factors such as implant fracture, overloading, and implant-abutment separation. Implant fracture is a catastrophic event, and implant-abutment separation or space at the interface-in addition to the mechanical complications-provides a focus for pathogenic microbes around the implant.30 Microbial factors compromise implant sites and are associated with both short- and long-term failures.
Short-term implant failure occurs during the healing phase when the implant fails to osseointegrate. Primary implant stability at the time of placement is important and enables the implant to successfully osseointegrate.31,32 Overheating of bone and microbial contamination during surgery result in early implant failure.33 Using a sterile and atraumatic surgical technique, minimizing contact with saliva at the site, and avoiding contamination of the sterile implant by glove powder or contact with gloves (which is associated with biofilm development)34 minimizes the potential for microbial contamination. The other nonsystemic influence is the level of oral hygiene the patient achieves.
Long-term implant failure involves the microbial environment and disease processes of peri-mucositis and peri-implantitis, which are analogous to gingivitis and periodontitis in the natural dentition. A retrospective study of ITI implants found a long-term failure rate due to infection of 0.6 percent per year over an eight-year period.35
Inflammation of the soft tissue around an implant (peri-mucositis) is reversible, while peri-implantitis involves loss of the supporting bone. Once inflammation of the peri-implant soft tissue has progressed to degradation of connective tissue and bone resorption, the disease has worsened to irreversible peri-implantitis. When implant patients experimentally refrained from oral hygiene for three weeks, the microbial composition around both natural teeth and implants consisted of similar percentages of spirochetes and motile rods.36 An increased number of P. intermedia, P. gingivalis, A. actinomycetemcomitans, and fusobacterium are found. In contrast to healthy implant sites, diseased implant sites are associated with a preponderance of gram-negative anaerobes. Implant failures have been associated with an anaerobic/aerobic ratio of 6:1.37 Finally, a further similarity appears to be the clustering of patients suffering from severe peri-implantitis or severe periodontitis; the risk of implant failure is 30 percent higher for patients who have already had an implant fail.38
Peri-implantitis is not normally associated with pain. Patients may be unaware of it unless there is an acute infection,39 underscoring how import it is for patients to diligently attend implant maintenance recalls to check that their home care is scrupulous and to ascertain that no peri-implant disease exists-or, if it does, to be able to intervene at an early stage in the disease progression.
Treatment of peri-implantitis may begin with mechanical removal of plaque and debris and effective home care. Peri-mucositis can be treated and reversed by this, but left untreated it progresses to irreversible peri-implantitis. Treatment of peri-implantitis involves mechanical removal of the bacteria, a reduction in bacterial colonies on the implant, and altering the microbial flora to a lower content of anaerobes.40 Following this, bone regeneration may be indicated depending upon the amount of bone lost and the patient response and care.
Prior to treatment of peri-implantitis, oral hygiene must be improved to reduce the number of pathogens. At the time of surgery, granulation tissue and toxins must be removed from the implant. It has also been recommended that the patient rinse twice daily with 0.12 percent chlorhexidine following surgery and receive antibiotics.
Recommendations for detoxification and cleansing of the implant include the use of hydrogen peroxide;41 supersaturated citric acid followed by saline;42 or, alternately, gauze soaked in chlorhexidine and saline.43 Decontamination of implants by a diode soft laser (690nm for 60 seconds) has been shown to be effective by Dortdubak et al. Surgical treatment of implants with a rough textured surface produces better results. The use of sustained release doxycycline (Atridox) as an adjunct in peri-implantitis treatment, together with implant scaling and oral hygiene instruction, has been shown to reduce probing depths and result in a greater attachment level gain.44 The use of 25 percent metronidazole dental gel (Elyzol Cabon) has been shown in Europe to decrease gram-negative organisms by 60 to 70 percent and to increase gram-positive organisms by 40 to 50 percent, providing for a good recovery.45 Despite careful treatment, advanced peri-implantitis can ultimately lead to implant removal.
Controlling the Intraoral Environment
Presurgically
Treatment of pre-existing caries and infections improves treatment outcomes. Periodontal disease should be treated prior to embarking on implant surgery to reduce the presence of periodontal pathogens. Smoking cessation prior to surgery and during healing has been found to reduce the rate of implant failure in (former) smokers. Cessation of smoking resulted in fewer short-term failures during the healing phase-the failure rate mimicked that of nonsmokers, and long-term failures were fewer than for those who did not stop smoking.46
Prophylactic antibiotics have been recommended at the time of implant surgery for all patients to help prevent peri-implant infections,47 while others recommend that antibiotics be considered specifically for diabetics48 and at-risk patients with systemic considerations (including immune-compromised patients and those who require prophylactic antibiotics for conditions such as cardiac defects). Quirynen et al. found no difference in early failure rates with or without prophylactic antibiotics,49 and found early failure to be associated with contamination at the time of surgery and high plaque and gingival indices. This further emphasizes the need for scrupulous oral hygiene by patients prior to implant surgery and its consideration in patient selection.
Preprocedural rinsing may reduce the oral flora. Veksler et al. found saliva samples taken immediately after, 30 minutes after and 60 minutes after two 30-second preprocedural rinses with 0.12 percent chlorhexidine gluconate resulted in reduced salivary bacterial loads of 97 percent, 77 percent, and 96 percent, respectively, versus baseline.50 Other studies have found preprocedural rinsing to be of little benefit. On balance, it would seem to be a reasonable measure that may benefit patients.
Postsurgically
Exposure of the implant to the intraoral environment will either be immediate or follow exposure of an implant that was submerged while osseointegration occurred (a one-stage versus a two-stage procedure). Either way, good oral hygiene is necessary to aid healing and the only difference is the earlier exposure of one-stage implants to pathogenic bacteria. However, this does not influence the long-term success of implants, and submerged implants acquire the same microbiota as the surrounding areas shortly after exposure to the intraoral environment. Using an adjunctive antimicrobial rinse will help reduce the microbial load and may be particularly helpful while the area may be uncomfortable following surgery. Use of an alcohol-free rinse should be considered as it is nonirritating to the healing mucosa. Use of an alcohol-free CHX (Gum®, Sunstar Butler) provides the benefits of 0.12% chlorhexidine rinse, and its use avoids the burning sensation and dry mouth that may be associated with the use of alcohol-containing mouthrinses.
Maintenance Phase
Regular evaluation of implants is necessary to ensure that patients are continuing to maintain scrupulous oral hygiene around dental implants and to reinforce with patients the need to do so. Evaluation should include an assessment of the health and integrity of soft and hard tissues around the implant51 every three to four months. Peri-implantitis can be detected at recalls by checking for increases in probing depths that may or may not be associated with bleeding and suppuration. An occlusal analysis is required to evaluate the interproximal contacts and the occlusal pattern of the prosthesis. Radiographic assessment is required to identify and evaluate any bony defect. After the first year of osseointegration, the implant should maintain a steady state level of bone.52
Oral Hygiene and Adjunctive Measures
Brushing and Flossing
Studies have also found that the use of either a manual or a powered toothbrush is safe and effective for patients with implants. One study followed 100 patients, and found that there was a very slight decrease in probing depth (from 3.3mm down to 3.0mm) with no adverse events associated with use of an electric toothbrush.53 However, a review using the Cochrane Database assessed all randomized clinical trials and found no evidence that powered or sonic toothbrushes were superior to manual toothbrushing.54
Recent research has shown that toothbrushes and toothpastes can affect the surfaces of titanium implants,55 and it is recommended that a low-abrasion toothpaste and soft-bristled toothbrush be used for oral hygiene procedures. Toothbrushes have been designed for implant patients (e.g., TePe Implant Toothbrush), and toothbrushes are available with ultra-soft 4mm diameter bristles (Delicate Toothbrush, Sunstar Butler).
Brushing and flossing are both crucial, yet clinical experience has shown that many patients almost never floss around implants.56 Patient education and motivation to floss around implants cannot be overemphasized. Implant hygiene kits are available and may help by providing patient convenience with the oral hygiene aids required together in one kit.
While flossing around implants requires a different technique from that used when flossing around natural teeth, flosses have been specifically designed for implants to make this easier (Postcare®, Sunstar Butler; Thornton Bridge and Implant Cleaners, Thornton; Superfloss®, Oral B®). Patients must floss under the mucosal cuff surrounding the implant. Interdental brushes have been found to be effective in cleaning under overdenture bars as well as around the implant mucosal cuff. The thickness of the entire gingival soft tissue and of the keratin layer in peri-implant mucosa has been found to be 34 percent and 50 percent thinner, respectively, than in healthy mucosa.57 Consequently, all kinds of products from the oral cavity and peri-implant pocket easily penetrate peri-implant mucosa. Given these facts, scrupulous oral hygiene is required around retained natural teeth by implant patients. In the absence of systematic supportive treatment and diligent oral hygiene, peri-implantitis is a common occurrence.58
Adjunctive Oral Hygiene
In one controlled trial, the use of an essential oil mouthwash twice a day for 30 seconds as an adjunct to implant-appropriate oral hygiene habits resulted in a 54 percent plaque reduction compared to the use of a placebo mouthrinse, and a 34 percent reduction in bleeding.59
Chlorhexidine gluconate rinses offer substantivity with slow intraoral release and have been used both preventively and therapeutically as a rinse, gel, or subgingival irrigation solution in a number of trials to control the intraoral environment and microbial load around implants. Morris et al. found that rinsing twice daily with 0.12 percent chlorhexidine improved the success rate of implants in type 2 diabetics by 9.1 percent and by 2.5 percent in nondiabetic patients.60 Rinsing with chlorhexidine as part of a one-stage full-mouth disinfection procedure in patients undergoing periodontal treatment has been found in several studies to result in a significant reduction in the numbers and presence of periodontal pathogens, a higher probing-depth reduction, and more attachment gain.61,62,63 De Soete et al. found that one-stage full-mouth disinfection resulted in an improvement compared to the control group during eight months of treatment and that P. gingivalis and B. forsythus were undetectable.64 All of these studies were conducted on patients with periodontitis rather than peri-implantitis. Considering the microbial similarities between periodontitis and peri-implantitis, it would seem reasonable to apply this chlorhexidine rinsing regimen to implant patients. Use of an alcohol-free chlorhexidine gluconate rinse avoids the side effects associated with alcohol-containing rinses.
Partially dentate patients at risk for caries will benefit from home fluoride therapy and professionally applied topical fluorides. Acidulated phosphate fluoride application alters the morphology of titanium and titanium alloys and has been shown to corrode titanium alloys at concentrations of 0.05 percent. At 2 percent APF, increased dissolution of titanium occurs,65 which supports earlier work by Probster et al. concerning interaction of APF and titanium surfaces. Therefore, when home fluoride therapy or professional fluoride applications are required in partially dentate implant patients, the use of neutral sodium fluoride is recommended.
Instrumentation Around Implants
Metal instruments have been found to have the potential to roughen the surface of the neck of titanium implants, with negative implications for increased plaque formation, retention, and maturation.66 In vitro studies have found that titanium alloy and stainless steel-tipped curettes and metal ultrasonic scalers roughen the surface of titanium implants. The use of air-abrasion polishing is contraindicated around implants due to the risk of marginal bone loss.67
Plastic scaling instruments, floss, polishing cups and paste and interdental brushes have all been found to be safe for implants, as have plastic-tipped ultrasonic scalers.68,69,70 In vivo testing has shown plastic scalers, rubber cups and pumice, and toothbrushes to be safe for implants and to not alter the surface roughness.
Summary
Implant therapy is a successful and well-accepted treatment modality. Implant treatment success is multifactorial, including both systemic and local factors. By controlling the microbial environment intraorally, implant success rates increase. Once a patient receives an implant, the single most important long-term determinant for implant success is diligent oral hygiene and home care by the patient. Brushing and flossing are critical success factors, requiring considerable patient education and motivation, and adjunctive therapy may be helpful. With careful patient selection and patient commitment to oral hygiene measures, the potential for long-term success and implant health is excellent.
References
1. Klinge N, Hultin M, Berglundh T. Peri-implantitis. Dent Clin N Am 2005;49:661-676.
2. Skaleric U, Schara R, Medvescek M, Hanlon A, Doherty F, Lessem J. Periodontal treatment by Arestin and its effects on glycemic control in type 1 diabetes patients. J Int Acad Periodontol. 2004 Oct;6(4 Suppl):160-165.
3. Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent 2000;20:366-373.
4. Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000 Dec;5(1): 157-165.
5. Sugarman PB, Barber MT. Patient selection for endosseous dental implants: oral and systemic considerations. Int J Oral Maxillofac Implants 2002;17:191-201.
6. Wolfaardt J, Granstrom G, Friberg B, Jha N, Tjellstrom A. A retrospective study on the effects of chemotherapy on osseointegration. J Facial Somato Prosthet 1996;2:99-107.
7. Schwartz HC. Osteonecrosis and bisphosphonates: correlation versus caution. J Oral Maxillofac Surg 2004; 62: 763-764.
8. Greenberg MS. Intravenous bisphosphonates and osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004; 98.
9. Schwartz HC. Osteonecrosis and bisphosphonates: correlation versus caution. J Oral Maxillofac Surg 2004; 62: 763-764.
10. Compendium, Vol. 18, No. 2, p 55, 1997.
11. Oral Health in Cancer Therapy. A Guide for Health Care Professionals. 1999; 22-23.
12. Sugarman PB, Barber MT. Patient selection for endosseous dental implants: oral and systemic considerations. Int J Oral Maxillofac Implants 2002;17:191-201.
13. Al-Nawas B, Grotz KA. Prospective study of the long-term change of the oral flora after radiation therapy. Support Care Cancer. 2006 Mar;14(3):291-296. Epub Nov 2005.
14. Nordereyd O, Hugoson A, Grusovin G. Risk of severe periodontal disease in a Swedish adult population. A longitudinal study. J Clin Periodontol 1999;26:608-615.
15. Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol 1996;1:1-36.
16. Porter JA, von Fraunhofer JA. Success or failure of dental implants? A literature review with treatment considerations. Gen Dent 2005;53(6): 423-432.
17. Bain CE, Moy PK. The association between the failure of dental implants and cigarette smoking. Int J Oral Maxillofac Implants 1993;8:609-615.
18. Haas R, Haimbock W, et al. The relationship of smoking on peri-implant tissue: a retrospective study. J Prosthet Dent 1996;76:592-596.
19. Lindquist I, Carlsson G, Jemt T. Association between marginal bone loss around osseointegrated mandibular implants and smoking habits: a 10-year follow-up study. J Dent Res 1997;76:1667-1674.
20. Galindo-Moreno P, Fauri M, Avila-Ortiz G, Fernandez-Barbero JE, Cabrera-Leon A, Sanchez-Fernandez E. Influence of alcohol and tobacco habits on peri-implant marginal bone loss: a prospective study. Clin Oral Implants Res. 2005 Oct;16(5):579-586.
21. Porter JA, von Fraunhofer JA. Success or failure of dental implants? A literature review with treatment considerations. Gen Dent 2005;53(6): 423-432.
22. Bryant SR. The effects of age, jaw site, and bone condition on oral implant outcomes. Int J Prosthodont 1998;11:470-490.
23. Porter JA, von Fraunhofer JA. Success or failure of dental implants? A literature review with treatment considerations. Gen Dent 2005;53(6): 423-432.
24. Chen S, Darby I. Dental implants: Maintenance, care and treatment of peri-implant infection. Aus Dent J 2003;48(4):212-220.
25. Porter JA, von Fraunhofer JA. Success or failure of dental implants? A literature review with treatment considerations. Gen Dent 2005;53(6): 423-432.
26. Klinge N, Hultin M, Berglundh T. Peri-implantitis. Dent Clin N Am 2005;49:661-676.
27. Quirynen M, de Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Impl Res 2002;13:1-19.
28. Shaffer MD, Juruaz DA, Haggerty PS. The effect of periradicular endodontic pathosis on the apical region of adjacent implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998 Nov;86(5):578-81.
29. Quirynen M, Vogels R, Alsaadi G, et al. Predisposing conditions for retrograde peri-implantitis, and treatment suggestions. Clin Oral Implants Res 2005;16(5):599-608.
30. Quirynen M, Bollen CML, Eyssen H, van Steenberghe D. Microbial penetration along the implant components of the Branemark system. An in vitro study. Clin Oral Implants Res 1994;5:239-244.
31. Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998 Sep-Oct;11(5):491-501.
32. Lioubavina-Hack N, Lang NP, Karring T. Significance of primary stability for osseointegration of dental implants. Clin Oral Implants Res. 2006 Jun;17(3):244-250.
33. Quirynen M, de Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Impl Res 2002;13:1-19.
34. Clarizio LF. Peri-implant infections. Oral and Maxillofacial Infections 2000;8(1):35-54.
35. Buser D, Merickse-Stern, et al. Long-term evaluation of nonsubmerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res 1997;8:161-172.
36. Pontoriero R, Tonetti MP, Carneale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res 1992;3:1-8.
37. Mombelli A, van Oosten MAC., Schurch E, Lang NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol and Immunol 1987;2:145-151.
38. Weyant RJ, Burt BA. An assessment of survival rates and within-patient clustering of failures of endosseous oral implants. J Dent Res 1993;72:2-8.
39. Chen S, Darby I. Dental implants: Maintenance, care and treatment of peri-implant infection. Aus Dent J 2003;48(4):212-220.
40. Quirynen M, de Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Impl Res 2002;13:1-19.
41. Leonhardt A, Dahlen G, Renvert S. Five-year clinical, microbiological and radiological outcome following treatment of peri-implantitis in man. J Periodontol 2003;74:1415-1422.
42. Clarizio LF. Peri-implant infections. Oral and Maxillofacial Infections 2000;8(1):35-54.
43. Schou S, Berglundh T, Lang NP. Surgical treatment of peri-implantitis. Int J Oral Maxillofac Implants 2004;19 Suppl:140-149.
44. Buchter A, Meyer U, Kruse-Loser B, et al. Sustained release of doxycycline for the treatment of peri-implantitis: randomized controlled trial. Br J Oral Maxillofac Surg 2004;42(5):439-444.
45. Stellini E, Migliorato A, et al. Topical treatment of peri-implantitis with metronidazole dental gel 2%. Clinical analysis and microbiological control. Minerva Stomatol 2000;49(1-2):59-67.
46. Bain C. Smoking and implant failure - benefits of a smoking cessation protocol. Int J Oral Maxillofac Implants 1996;11:756-759.
47. Clarizio LF. Peri-implant infections. Oral and Maxillofacial Infections 2000;8(1):35-54.
48. Sugarman PB, Barber MT. Patient selection for endosseous dental implants: oral and systemic considerations. Int J Oral Maxillofac Implants 2002;17:191-201.
49. Quirynen M, de Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Impl Res 2002;13:1-19.
50. Veksler AE, Kayrouz, GA, Newman MG. Reduction of salivary bacteria by preprocedural rinses with chlorhexidine 0.12%. J Periodontol. 1991 Nov;62(11):649-651.
51. Palmer RM, Pleasance C. Maintenance of osseointegrated implant prosthesis. Dent Update 2006; 33(2):84-86.
52. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11-25.
53. Vandekerckhove B, Quirynen M, Warren PR, Strate J, van Steenberghe D. The safety and efficacy of a powered toothbrush on soft tissues in patients with implant-supported fixed prosthesis. Clin Oral Investig 2004;8(4): 206-210.
54. Esoposito M, Worthington HV, Thomsen P, Coulthard P. Interventions for replacing missing teeth: maintaining health around dental implants. Cochrane Database Syst Rev 2004;3:CD003069.
55. Hossain A, Okawa S, Miyakawa O. Effect of toothbrushing on titanium surface: an approach to understanding surface properties of brushed titanium. Dent Mater. 2006 Apr;22(4):346-352. Epub 2005 Jul 27.
56. Clarizio LF. Peri-implant infections. Oral and Maxillofacial Infections 2000;8(1):35-54.
57. Lindhe J, Berglundh T. The interface between the mucosa and the implant. Periodontol 2000. 1998;17:47-54.
58. Roos-Jansaker AM, Lindahl C, Renvert H, Revert S. Nine-to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol 2006;33(4):290-295.
59. Esoposito M, Worthington HV, Thomsen P, Coulthard P. Interventions for replacing missing teeth: maintaining health around dental implants. Cochrane Database Syst Rev 2004;3:CD003069
60. Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000 Dec;5(1): 157-165.
61. Bollen CM, Mongardini C, Papaioannou W, Van Steenberghe D, Quirynen M. The effect of a one-stage full-mouth disinfection on different intraoral niches. Clinical and microbiological observations. J Clin Periodontol. 1998 Jan;25(1):56-66.
62. Mongardini C, van Steenberghe D, Dekeyser C, Quirynen M. One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. I. Long-term clinical observations. J Periodontol. 1999 Jun;70(6):632-645.
63. Quirynen M, Mongardini C, Pauwels M, Bollen CM, Van Eldere J, van Steenberghe D. One-stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. II. Long-term impact on microbial load. J Periodontol. 1999 Jun;70(6):646-656.
64. De Soete M, Mongardini C, Peuwels M, Haffajee A, Socransky S, van Steen-berghe D, Quirynen M. One-stage full-mouth disinfection. Long-term microbiological results analyzed by checkerboard DNA-DNA hybridization. J Periodontol. 2001 Mar;72(3):374-382.
65. Matono Y, Nakagawa M, Matsuya S, Ishikawa K, Terada Y. Corrosion behavior of pure titanium and titanium alloys in various concentrations of Acidulated Phosphate Fluoride (APF) solutions. Dent Mater J. 2006 Mar;25(1):104-112.
66. Romeo E, Ghisolfi M, Carmagnola D. Peri-implant disease. Minerva Stomatol 2004;53:215-230.
67. Bergendal T, Forsgren L, Kvint S, Lowstedt E. The effect of air abrasive instrument on soft and hard tissues around osseointegrated implants. Swed Dent J 1990;14:219-223.
68. Matarasso S, Quaremba G, Coraggio F, Vaia E, Cafiero C, Lang NP. Maintenance of implants: an in vitro study of titanium implant surface modifications subsequent to the application of different prophylaxis procedures. Clin Oral Implants Res. 1996 Mar;7(1):64-72.
69. Fox SC, Moriarty JD, Kusy RP. The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol 1990;61:485-490.
70. Sato S, Kishida M, Ito K. The comparative effect of ultrasonic scalers on titanium surfaces: an in vitro study. J Periodontol 2004;75(9):1269-1273.
Author Profile
Richard Nejat, DDS, Clinical Assistant Professor, Department of Implant Dentistry, Department of Periodontology, New York University
Dr. Richard Nejat is board certified by the American Board of Periodontology. His practice is on the forefront of computer guided implant dentistry and minimally invasive dental surgery. He is a course instructor in various continuing educational seminars, symposiums to colleges, and dental societies. Dr. Nejat attended Drew University and continued his education at New York University, where he attained his doctorate of dental surgery. Dr. Nejat was elected to membership in Omicron Kappa Upsilon, the National Dental Honor Society recognizing academic and clinical excellence in dentistry. He earned his certificate in periodontics from the State University of New York at Stony Brook. Dr Nejat is currently clinical assistant professor in the department of implant dentistry and periodontics at New York University and maintains private practices in Manhattan and Nutley, New Jersey.
Daniel Nejat, DDS
Dr. Daniel Nejat completed his college education at Drew University, where he graduated magnum cum laude. He attained his doctorate of dental medicine at the University of Medicine and Dentistry of New Jersey Dental School. He earned his certificate in periodontics from New York University College of Dentistry. Dr. Nejat is currently practicing periodontal and implant dentistry in New York City.
Fiona M. Collins, BDS, MBA, MA
Dr. Fiona M. Collins has over 20 years of clinical, marketing, education and training, and professional relations experience. She has practiced as a general dentist for 13 years, written and given CE courses to dental professionals and students, and conducted market research projects. Dr. Collins is a past member of the Academy of General Dentistry Health Foundation Strategy Board and has been a member of the British Dental Association, the Dutch Dental Association, and the American Dental Association. Dr. Collins earned her dental degree from Glasgow University and holds an MBA and MA from Boston University.
Disclaimer
This course has been made possible through an unrestricted educational grant. The authors of this course have no commercial ties with the sponsors or the providers of the unrestricted educational grant for this course.
Reader Feedback
We encourage your comments on this or any ADTS course. For your convenience, an online feedback form is available at www.ineedce.com.
Click here to view Intoral Course Questionnaire...