New Product Development: The role of the dental hygienist
Hygienists use research skills to gather data to ensure the launch of an innovative product.
by Katherine Ortblad, RDH, MHA, and Camille Baltuck, RDH, BS
A stroll through the dental care section of a drugstore or supermarket clearly demonstrates the wide array of consumer products targeting the oral care needs of consumers. Many of the products are labeled “new” or “improved.” Companies continually refine existing products to enhance performance, or they develop new products in order to maintain their existing customer base or capture a different segment of the consumer market. What these “new” or “improved” products offer to the consumer may range from a new taste sensation to a new application of technology - a new flavor of mouthwash or the addition of ultrasound to a power toothbrush.
Companies often apply significant “people-power” to these improvements, sometimes consuming years of research effort. Improving existing products and developing new oral care products are not simple processes. These processes, one may be surprised to find, often utilize the input of trained dental professionals.
The typical development process for a product works through a systematic series of investigations and designs that aim for the ultimate goal of a product that can be marketed. In developing new oral care products, a team of professionals with different areas of expertise is brought together. Such a team often has a product feature or technology application that is the focus of a development effort. Several iterations through the design-build-test cycle yield an improved product after each cycle, one step closer to the final product to be marketed.
Within the oral care industry, the development team may include engineers, scientists, and dental professionals. The dental professional is often focused on ensuring the product’s safety, efficacy, and utility for the consumer.
The dental professional’s role is critical for the success of the product, particularly if the technology is revolutionary in innovation. Dentists and dental hygienists understand dental anatomy, as well as patient and clinician concerns. If hired from a traditional private practice setting, they offer valuable insight during the development of a new product. The dental hygienist, who is the primary provider of oral hygiene information, can provide key insight into understanding patient behavior and motivation. These insights are especially important in the design and test portions of the development cycle.
Ultreo, Inc. recently used dental hygienists’ expertise, for example, in an industrial setting to help with the development of a home-care product. The company’s development cycle led to the recent market introduction of an ultrasound toothbrush. Input from dental professionals was applied from the inception of the product’s concept. When the product was sufficiently complete to allow testing with human subjects, a research dental hygienist was hired by Ultreo.
This article discusses the use of dental hygiene personnel in a team of professionals developing a revolutionary new oral care product. As will be discussed, pilot studies conducted as part of product development allow the design of larger independent clinical studies.
Testing, Testing, Testing
The research dental hygienist, in this example, was a member of the clinical assessment team. The team played an important role in establishing the testing capabilities for the company. In a start-up environment, these capabilities must be established from the ground up.
The first priority is to assess the company’s testing needs. What parts of development and/or testing should be handled internally? What outside resources such as contract research organizations (CRO) or university-based research groups should a company use? The assessment includes determining the type of data to be gathered (through questionnaires or clinical measurement of short-term or long-term use, etc.).
If the choice is to develop internal testing capabilities, this requires setting up a research facility. The research dental hygienist’s expertise in setting up a facility for the examination and treatment of patients is important:
- Clinical evaluations with human subjects requires a fully functional clinic environment following applicable health and safety codes.
- Basic plaque and gingivitis studies require a dental chair with water and air for effective rinsing and drying the tooth and tissue surface prior to evaluation.
- Gingival health studies may require additional equipment for assessing gingival crevicular fluid.
- Procedures involving the collection of specimens require immediate assessment capabilities or a means of storage of the specimen (e.g., cryogenic freezer or liquid nitrogen).
The research dental hygienist can ensure that the company follows requirements relating to the use of human subjects. Such studies require approval of the research methodologies by an institutional review board (IRB). All personnel involved in human studies need to complete applicable training conforming to the Belmont Report, or other applicable standards. It is also helpful if the personnel are trained in good clinical practices (GCP). Standard operating procedures must be put in place regarding critical procedures that must be followed. The research dental hygienist may be involved in writing protocols and procedures, handling communication with the IRB, and training new personnel.
Product development requires a flexible research environment. Ultimately, to establish product claims, independent, randomized, controlled and blinded clinical evaluations are important. However, all of these may not be practical in the early phases of a product development cycle. All of the variables in conducting and gathering reliable data requires the clinical assessment team to be aware of both the strengths and the limitations of pilot studies.
The research dental hygienist’s role in conducting studies is multifaceted. In preparation, subjects are recruited, screened, consented, and scheduled. Additionally, prior to initiating a study, the hygienist collaborates with the engineering team to assure that the product has been appropriately designed, adequately tested, and prepared for clinical use.
Conducting Pilot Studies
Ultreo’s toothbrush, which shares the same name as the company, underwent numerous pilot studies. Prior to clinical testing, safety factors regarding sonic and ultrasonic activity initiated in the oral cavity by the prototype had to be considered. During the first clinical study, a more extensive evaluation of adverse effects on gingival tissue, for example, was recorded.The research dental hygienist’s comparison of the data suggested no areas of concern and that it was safe to continue with additional short-term clinical evaluations.
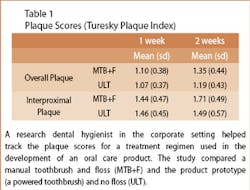
The clinical assessment team then focused on a series of efficacy evaluations (randomized, controlled, and examiner-blinded). For example, the team initiated and completed a study where each subject participated in two 2-week treatment regimens: a manual toothbrush and dental floss, or Ultreo and no dental floss (see Table 1).
From there, the research dental hygienists participated in the data interpretation that aided the clinical assessment team in designing subsequent larger clinical studies.
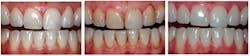
During the efficacy studies, questionnaires designed by the research dental hygienist gathered feedback from users. An example of how comments fueled additional studies was the subjects’ perception that teeth appeared whiter after using Ultreo.
So Ultreo piloted a study involving extrinsic stain removal (see Figure 1). Observations confirmed the ability of the prototype toothbrush to remove extrinsic stain, again providing the clinical assessment team valuable insight into the methodology for subsequent studies.
Involvement In Industry
In developing a new dental product, each member of the product development team has an important and defined role. The clinical assessment team is focused on the needs of the consumer, ensuring the product’s safety, efficacy, and utility.
The research dental hygienist has very defined responsibilities including, but not limited to, designing protocols, executing studies, interpreting data, and writing reports.
The close interaction between the user of the prototype toothbrush and the research dental hygienist provides data for product refinement as well as indications of product performance. The researchers identified behavioral needs - particularly from the aspect of oral hygiene - and the valuable information was collected and applied to the development of the Ultreo. By talking with and observing volunteers, benefits not originally identified surfaced and areas of potential concern were identified.
Data captured though pilot studies - examples of which have been briefly described above - provided feedback on the product’s performance.
Trends in data also suggest whether a larger independent study conducted at a university or CRO is warranted. Pilot data is important for protocol refinement, including sample size determination, expected outcome differentials, effective study duration and whether certain clinical measures or indices are appropriate. The outcome of pilot studies may also indicate the location of where an independent study should be conducted, depending, in part, on the variables to be tested and the expertise of the independent research site.
Effective use of pilot studies is important where financial resources may be limited, particularly in a start-up company. Insight gained in the execution of pilot studies may allow the research dental hygienist to effectively implement, and potentially monitor, external independent studies.
Knowledge of GCP guidelines is critical for the execution of clinical studies, whether internally conducted or executed at an independent site. Independent studies are typically used by companies to support product claims.
The role of the dental hygienist in new product development not only involves the dental hygienist as a researcher but also as an educator and advocate for the product in terms of professional relations.
The dental hygienist is involved in many different aspects of industry. As an educator the dental hygienist teaches proper use of the product and educates other professionals on the benefits of new innovative products. As an advocate for the product, the dental hygienist networks with other dental professionals, educates them on the new technology, and encourages them to recommend and incorporate the new product into their clinical practices.
New or improved oral care products provide consumers with many choices for their home oral-care regimen. The role of the dental hygienist in developing many of these products is significant. The dental hygienist is an important member of the professional team assembled by companies in developing improved products and bringing new technologies to the market.
From the inception of the product idea to the launch of a new product and beyond, successful oral care companies use the dental hygienist each step of the way. The dental hygienist’s involvement is key to establishing a product’s safety, efficacy, and usefulness to the consumer.
About the Authors
Katherine Ortblad, RDH, MHA, and Camille Baltuck, RDH, BS, are both employed by Ultreo, Inc., in Redmond, Washington, as researchers. Katherine and Camille each have over 15 years research experience, both in industrial and academic environments. Prior to working for Ultreo, Inc., both were employed by Philips Sonicare and had worked in private practice.