Scary, nasty, and shocking: The risks associated with instrument retipping
What does a hygienist purchase when he or she purchases a new dental scaler, explorer, probe, curette, or file? What thought process went into the creation of the instrument? What intricacies set that instrument apart from other instruments that can be perceived to "all do the same thing"? What happens when the standards of manufacturing these medical devices are breached, and the instrument is altered in shape, quality, balance, size, and materials? Do these changes affect the disinfection and sterilization standards our offices have in place to ensure the safety of our patients? These were my concerns as I dug deeper to find what I didn't know about instrument retipping.
Hygienists know that the tactile sensitivity of the dental hand instrument reigns supreme for ensuring proper calculus removal both supra- and subgingivally. The FDA has set standards for the use of dental instruments as medical devices, and states an instrument is a medical device because it "is intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes." Dental professionals should take every practical step in ensuring the sterile maintenance and handling of these instruments to protect the safety of their patients.
Various safety measures are in place prior to their release to consumers. Each manufacturer is encouraged to comply with ISO standards for quality control. There are specific procedures that must be followed for quality assurance standards, both during testing and in the production facility.
Although there is no mandated standard for the materials used in surgical medical-grade steel manufacturing, each company has a specific recipe for the quality of steel used. Some manufacturers even have multiple recipes. Carbon, chromium, nickel, and molybdenum all provide specific properties to the steel. The carbon, nickel, and molybdenum all contribute to the hardness of the steel through a heat-treated process. Proper heat treating is necessary to provide the steel with the necessary properties to maintain a long-lasting cutting edge, while maintaining adequate resistance to breakage. Chromium provides the proper corrosion resistance to withstand sterilization. The cutting edge is the most valuable part of the dental instrument, and while many other portions of the instrument have standards and controls, the shank shape and cutting edge are what set each instrument apart from others in the industry. No clinician wants rusty, corroded instruments in their patient cassette, so these proprietary recipes are of great significance to each dental instrument manufacturer. In addition, altering or repeating the heat treatment during the course of retipping can result in a completely altered instrument.
Each instrument that leaves the production facility has consistency in balance, size, shape, and sharpness. The balance of the instrument is what ensures each instrument feels similar in the clinician's hand, allowing the user to have consistency in the amount of pressure needed in the working stroke. This factor is what we as clinicians use in determining if we prefer a resin handle or a stainless steel handle, and whether we want that handle to be round, hexagonal, gnarled, or tapered. Assuring the handle is consistent each time the clinician removes the instrument from the tray is part of the manufacturer's goals in production. Additionally, the size, shape, and sharpness reliability is crucial. Most clinicians have multiple cassettes of instruments, and prefer each cassette to be consistent so there is no time wasted in searching for an instrument during patient chair time.
Quality construction and consistency is critically important with scalers and curettes, as they are classified as medical devices -- instruments intended for use in the diagnosis of disease or other conditions, or in the curing, mitigation, treatment, or prevention of disease.
Reputable instrument manufacturers are extremely concerned with the construction integrity of their instruments and will stand behind the quality of their instruments, as customer satisfaction is paramount. One should check to see what sort of warranty instrument manufacturers offer, as some offer warranties for the expected useful life of an instrument.
Clinicians are often curious about the life expectancy of scalers and curettes, but there is no single precise answer that can be given. The life expectancy of an instrument is influenced by a wide variety of factors including the number of uses, instrument care and handling procedures, number of sharpenings and the sharpening technique, and types of patients seen in the operatory (including age and disease status). Consequently, the reasonable useful life of an instrument is determined by the clinician via regular evaluation of the working ends for changes in size and shape.
What happens when the reasonable useful life of the instrument is complete? Many companies have instrument recycling programs where clinicians can trade worn-out instruments for new ones. This data is available on each company's website. These programs offer an effective budget-saving method to reprocess unusable tools while also being kind to our ecological environment.
When the instrument is worn beyond the point of sharpening, or bent during inappropriate use, some clinicians choose to retip the instrument. But, what is retipping? Retipping is a process in which the original tip of the dental instrument is completely and forcibly removed from the handle through the breaking of chemical and mechanical processes of the original manufacturer adherence, and a new tip is pressed into the handle. This process involves heating and altering the original handle, and although high-quality metals are sometimes used to retip the instrument, the end product rarely meets the quality of the original piece. Therefore, no dental manufacturer in the United States will retip a dental instrument.
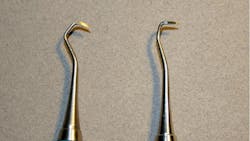
Retipped dental instruments do come at a significantly reduced cost, but with that reduced cost comes an increased risk to both the patient and clinician. A retipped dental instrument often has an altered balance and alignment. I handled many retipped instruments in researching this article. It became evident that the retipped instruments exhibited increased instability when held in the modified-pen grasp most clinicians prefer to use (Figure 1).
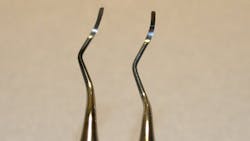
Another difference noted in evaluating retipped instruments was evidence of instability in the junction between the working tip and handle, some of which had noticeable gaps (Figure 3). When applying force to the long axis of the tooth in scaling or curetting motions, this discrepancy was noted while working on a typodont, which I would anticipate would be compounded when working in the mouth with natural tooth structure and gingiva to contend with as well. Clinicians depend on a solid working end when performing periodontal therapy subgingivally, and the last thing they want to question is the stability of their working end tip.
Other instruments had melted resin, which was noticeably reshaped (Figure 3b). When fabricating a resin handle, air voids can occur subsurface. In a reshaped resin handle, these air voids are a possible risk. Even in some of the surgical stainless steel retipped handles, a few of the evaluated instruments had cracks, weakening the overall attachment of metal to metal (Figure 4). If the force used to retip was great enough to crack the handle, or necessitate reshaping of the resin through melting, the strength and durability of the instrument is questionable to this researcher.
A primary concern in the use of retipped dental instruments is the collection of debris in the handle through visible and microscopic cracks, or gaps at the junction of the handle and working end, that can occur during the retipping process. Some of the retipped instruments evaluated had fluid from the ultrasonic cleaner that became encased within the handle, interfering with proper sterilization. Others had working end tips exhibiting significant corrosion after routine sterilization (Figure 5).
The angle of blades and original shank and cutting edge were noticeably altered in several retipped instruments. Some terminal shanks were so noticeably altered that I had difficulty rolling the instruments over a counter and attempting to position them on my typodont for assessment. Repeated retipping increases the chance for cracked handles and the risk that the tip may fall out during treatment. Although these retipped instruments had the original manufacturer's company logo on the handle, they were certainly not the cutting edges and shank bends I was used to grasping (Figures 6, 7a, 7b).
Prior to stumbling upon this topic last year in discussion with a colleague one evening over dinner, I did not know it was possible to have a dental instrument retipped, much less retipped more than once! The idea that a clinician would want to hire someone to take a medical device apart and then put it back together to create a new medical device that became a risk to the patient shocked me. Sure, it was a bit less costly short-term, but there are many more things more costly if a patient becomes harmed or infection control is breached in the care of armamentarium. The bottom line here is quality and time. Since I know that I can use less time to complete a procedure with a quality instrument, maintaining the consistency of care for my valuable patient, I choose not to retip. To retip, or not to retip? The choice is your own.
Editor's note: This article was originally published in 2013. Updated 2024.
References
Burns S, Bendit J. (2010) Proper instrument care: the myth of retipping. Incisal Edge.
Federal Register. (1996) "Medical devices: good manufacturing practice (CGMP) final rule; quality system regulation." FDA online: Accessed at http://www.fda.gov/medicaldevices/deviceregulationandguidance/postmarketrequirements/qualitysystemsregulations/ucm230127.htm.
Federal Register. (1997). Remanufactured devices: ensuring their safety and effectiveness. Medical Devices and Diagnostic Industry.
About the Author

Emily Boge, EdD, RDH, CDA, FAADH, FADHA, CDIPC
Wife, mother, farmer, college administrator, educator, inventor, public health advocate, businesswoman, researcher, writer, editor, speaker—yet always a dental hygienist—Emily Boge, EdD, RDH, CDA, FAADH, FADHA, CDIPC, has worn many hats over 20-plus years in the dental industry. She takes pride in utilizing her inquisitive mind and honest attitude to lead faculty at her college, influence manufacturers to listen to dental professionals in product innovation, and transform students into entry-level professionals, promoting the use of inner accountability, tenacity, and empowerment. Dr. Emily currently serves as dental administrative chair at Hawkeye Community College in Waterloo, Iowa, and owns a farm and the speaking, consulting, and writing firm Think Big Dental.