Editor's note: Originally posted in 2016; formatting reviewed and updated in 2023. Read "Selecting local anesthetic agents for nonsurgical periodontal therapy" by Laura Webb for more recent information.
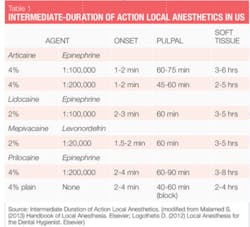
Nonsurgical periodontal therapy (NSPT) procedures are generally elective procedures requiring intermediate duration anesthetics (see Table 1). Everything that we need to provide safe, effective local anesthesia for our patients is available to us. So how do we choose? Selection of agents for NSPT should be based on patient profile, length of procedure, and the need for hemostasis. An overview of important agents (generic names) available in the United States is also discussed below, as well as their general considerations as they relate to NSPT with adult patients.
Agents and vasoconstrictors
All injectable dental local anesthetics available in North America today are amide local anesthetics. These agents are safe, nonallergenic, usually metabolized in the liver, and excreted by the kidneys (exceptions discussed later). They are also mild vasodilators, which result in an increased rate of anesthetic absorption into the bloodstream, an increased risk of systemic toxicity, reduction of duration of action, and increased bleeding in the area.
Vasoconstrictors are added to local anesthetic agents to counteract the vasodilatory properties. By constricting the blood vessels in the area, absorption is decreased, resulting in reduced risk of systemic toxicity, increased duration of action, and increased hemostasis.
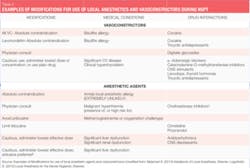
When providing local anesthesia, agents containing vasoconstrictors should be used unless there is a compelling reason or absolute contraindication not to use them.1 An absolute contraindication describes a circumstance when a drug should not be administered under any circumstances because it is unsafe. A relative contraindication describes a circumstance when the drug may be used carefully after thoughtful consideration of risk vs. benefit and when a safer alternative is not available. Most of the patients we treat fall into the latter category because there are few absolute contraindications to the administration of dental local anesthetic agents for patients who are eligible for elective procedures, such as NSPT (see Table 2).
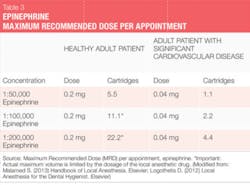
The two vasoconstrictors that are available in the U.S. are epinephrine and levonordefrin. Epinephrine is available in the dental anesthetics lidocaine, articaine, prilocaine, and bupivacaine. In U.S. dental cartridges, epinephrine is formulated in 1:50,000, 1:100,000, and 1:200,000 concentrations. It should be noted that the duration of effect for pulpal and soft tissue anesthesia is essentially the same with all these vasoconstrictor concentrations, and therefore the lowest concentration available is recommended. The 1:100,000 and 1:200,000 concentrations usually provide good hemostasis for NSPT. Agents with 1:200,000 epinephrine may be useful when it is necessary to limit vasoconstrictor dose (see Tables 2 and 3).1-3 The highest concentration, 1:50,000, is recommended only when additional hemostasis is required and should be administered in very small volume as infiltration (papillary) injections adjacent to site of bleeding.1-3 Levonordefrin is one-sixth as potent as epinephrine and is only available in 2% mepivacaine and 1:20,000 levonordefrin. It provides significantly less hemostasis than epinephrine and therefore is less useful for NSPT. Levonordefrin is absolutely contraindicated in patients who are taking tricyclic antidepressants.1 Sometimes, due to manufacturing issues, levonordefrin is not available.
As discussed above, there are few absolute contraindications for the use of a vasoconstrictor for patients eligible for NSPT. In most situations, limiting the amount of vasoconstrictor a patient receives produces an adequate benefit without compromising patient safety.
For example, patients with a relative contraindication for vasoconstrictors often may receive the lowest possible dose of epinephrine, not to exceed the "cardiac" maximum recommended dose of 0.04 mg per appointment (see Table 3). The risks of using epinephrine vs. the benefits should always be considered. Recall, though, that inadequate pain control may result in the release of unpredictable amounts of endogenous epinephrine, perhaps exceeding the dose that would be provided by the hygienist. The more medically compromised a patient is, the greater the need is for profound anesthesia.1,2
All dental local anesthetic cartridges with vasoconstrictors contain bisulfite preservatives. Bisulfites are also commonly found in food and beverages. Hypersensitivity to bisulfites has been reported, particularly with asthmatics (10% of asthmatics).2 Patients who have demonstrated a true allergy to bisulfites should not receive a local anesthetic agent containing vasoconstrictors (an absolute contraindication).
Specific agents
Lidocaine: Marketed in 1948, lidocaine was the first amide local anesthetic and a great improvement over the ester agents previously available. It remains the gold standard by which all others are judged and holds 49% of the U.S. market share.4 It is compounded with epinephrine as 2% lidocaine, 1:100,000 epinephrine and 2% lidocaine, 1:50,000 epinephrine. Lidocaine is absolutely contraindicated in patients with true allergy to amide type local anesthetics (extremely unlikely) or patients with known bisulfite allergy.
Mepivacaine: Marketed in 1960, it is available as 2% mepivacaine, 1:20,000 levonordefrin and 3% mepivacaine (plain). Mepivacaine has a milder vasodilatory effect than most other amides and so it may be useful with patients for whom vasoconstrictor is contraindicated and cannot receive 4% prilocaine plain.
However, the duration of action for 3% mepivacaine plain is short. Mepivacaine is absolutely contraindicated in patients with true allergy to amide type local anesthetics (extremely unlikely) or with the 1:20,000 levonordefrin formulation, patients with known bisulfite allergy or taking tricyclic antidepressants.
Prilocaine: Marketed in 1965, prilocaine is less toxic and less potent than lidocaine or mepivacaine and provides a slightly longer duration of action. It is available as 4% prilocaine 1:200,000 epinephrine and 4% prilocaine (plain).
An interesting feature regarding prilocaine plain is that not only does it have a milder vasodilatory effect than most other amides, but, when providing a block injection, it is the only intermediate duration plain local anesthetic. It can be a good choice for patients for whom vasoconstrictor is contraindicated. Both formulations of 4% prilocaine are recommended for patients with epinephrine sensitivity and requiring intermediate duration of action.
Prilocaine reduces the blood's oxygen-carrying capacity in higher doses (doses greater than maximum recommended dose) and, therefore, is relatively contraindicated for use with patients at risk for methemoglobinemia, patients with problems of oxygenation such as sickle cell anemia, cardiac/respiratory failure, and also for patients who are receiving acetaminophen or phenacetin because methemoglobin levels are increased.
Since prilocaine is also metabolized in the lungs and kidneys, it is metabolized more easily by the liver than lidocaine or mepivacaine. In addition, it clears the kidneys more rapidly than other the other amides.1 Prilocaine is absolutely contraindicated in patients with true allergy to amide type local anesthetics (extremely unlikely) and if using the 1:200,000 epinephrine formulation, in patients with known bisulfite allergy.
Articaine: Articaine has been available in Europe since 1976, but was not marketed in the United States until 2000. It is the second most popular local anesthetic in the U.S., currently holding 35.6% of the U.S. market share, and is the leading dental anesthetic in Canada and Europe.4
Its popularity has been attributed to higher injection success rates related to increased lipid solubility and faster diffusion through hard and soft tissues, including palatal root anesthesia with buccal injections and mandibular anesthesia with supraperiosteal injections.1,5-7 Reports also indicate more profound and longer duration of anesthesia.6
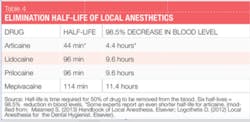
Classified as an amide with amide and ester characteristics, it is 1.5 times more potent than lidocaine and has similar toxicity. In the U.S., it is compounded with epinephrine as 4% articaine, 1:100,000 epinephrine and 4% articaine, 1:200,000 epinephrine. Biotransformation occurs both in the plasma and the liver. Because of its unique composition and biotransformation pathway, the elimination half-life (time required for 50% of a drug to be removed from the blood) of articaine, as reported by manufacturers, is only 44 minutes,4 more than twice as fast as all other amide agents, resulting in a decreased risk of system toxicity (see Table 4). This is significant, particularly, for patients for whom a higher rate of biotransformation may be desired (children, medically compromised, pregnant, nursing, liver disease, etc.). Some experts report that the elimination half-life of articaine is only 27 minutes (2.7 hours to decrease in blood level 98.5%)-even more rapid!1
Articaine is absolutely contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type, or in patients with known bisulfite allergy.
Other considerations
There has been some controversy about the use of 4% local anesthetics such as prilocaine and articaine with regard to increased neurotoxicity and increased risk of paresthesia.1,2,8,9 Only one study (Pogrel 2012) has been clinical in nature and was "based on patients actually seen and examined by a single clinician."9 In the Pogrel study, it was determined that the number of cases of paresthesia from articaine was proportional to its market share. Other reports have been retrospective in nature, reliant upon malpractice reports, and, therefore, may have been susceptible to reporting bias.
In a recent in vitro local anesthetic neurotoxicity study, it was concluded that articaine was the least neurotoxic and had the most favorable safety profile compared with lidocaine, mepivacaine, and prilocaine.8
Other experts assert that the paresthesia is most often related to mechanical trauma, not chemical trauma, because the lingual nerve is in the path of the sharp dental needle during provision of the IA block. This opinion is supported, in part, by the following:
• 95% of paresthesia cases occur in the mandible (usually the lingual nerve)
• There are no reports of paresthesia after provision of the Gow-Gates and Vazirani-Akinosi injections (where the lingual nerve is not in the vicinity)
• There are no reports of paresthesia after the use of articaine in medicine.10
Additional studies are needed, as the evidence is inconclusive regarding a greater risk of paresthesia with the use of articaine compared with the other local anesthetics.
We choose local anesthetic agents based upon our professional judgment, experiences, and the patient's profile. Information regarding the dosages, safety, and effectiveness of agents is constantly being updated. Continuous exploration of the scientifically based literature regarding local anesthetic agents is essential so we may ensure effective and safe provision of local anesthesia for our patients.
References
1. Malamed S. (2013) Handbook of Local Anesthesia. Elsevier.
2. Logothetis D. (2012) Local Anesthesia for the Dental Hygienist. Elsevier.
3. Schwartz PJ. (2013) Anesthesia, An Issue of Oral and Maxillofacial Surgery Clinics. Elsevier.
4. Septodont. www.septodont.com
5. Katyal V. The efficacy and safety of articaine versus lignocaine in dental treatments: a meta-analysis. J Dent. 2010 Apr; 38(4):307-17
6. Costa CG, Toramano IP, Rocha RG, Francischone CE, Totamano N. Onset and duration periods of articaine and lidocaine on maxillary infiltration. j.prosdent. 2005 Oct; 94(4): 381
7. Batista da Silva C, Berto LA, Volpato MC, Ramacciato JC, Motta RH, Ranali J, Groppo FC. Anesthetic efficacy of articaine and lidocaine for incisive/mental nerve block. J Endod. 2010 Mar; 36(3):438-41
8. Malet A, Faure, M, Deletage N, Pereira B, Haas J, Lambert G. The comparative cytotoxic effects of different local anesthetics on human neuroblastoma cell line. Anesthesia & Analgesia. 2015 Mar; 120(3):589-596
9. Pogrel MA. Permanent nerve damage from inferior alveolar nerve blocks-a current update. 2012 Oct; J CDA; 40(10):795-797
10. Malamed, S. A Renaissance in Local Anesthesia, International Seminars, May 2, 2015; Sacramento CA.