CHX: We don’t like it! Why are we still using it?
Herb Moskowitz, DDS
For decades, povidone iodine (PVP-I) and chlorhexidine gluconate (CHX) have established themselves as infection-control standards of care in hospitals throughout the world.1,2 For hospital use, CHX is used at either a 2% or 4% concentration. These “hospital-strength” concentrations contain 17 to 34 times more CHX than a 0.12% CHX oral rinse.
Although both CHX and PVP-I are good antiseptics, they are far from perfect. For example, both CHX and PVP-I have been the subject of several recalls due to bacterial contamination.3 It seems somewhat incongruous that antiseptics, which are supposed to protect us from germs, can actually be the breeding grounds for germs. In hospital use, CHX is often augmented with 70% isopropyl alcohol to achieve an adequate level of antimicrobial efficacy.4 Microbes can also develop resistance to CHX, which is a problem of growing proportions.5Dental professionals are all familiar with the shortcomings of CHX as an oral rinse. Staining; calculus formation; poor taste; altered taste; tissue irritation; potential carcinogenicity; flammability (due to its 11.6% ethyl alcohol content); short-term use restriction; and rare, but severe, anaphylactic shock come readily to mind.6 These shortcomings lead in turn to poor patient compliance. Although it has served an important role in periodontal care, CHX has only limited antiviral activity.7
A recent generational advance in iodine chemistry has led to a class of “super iodines” having important indications in dentistry and oral hygiene. This newly patented technology8 has none of the disadvantages of either CHX or PVP-I and is far safer and much more effective than either of them.9
PVP-I contains an equilibrium of several different types of the iodine molecule. The total of all the iodine present in povidone iodine is 31,600 parts per million (ppm). Of the 31,600-ppm total iodine present, only 1-3 ppm are molecular iodine (I2), the only type of iodine that is lethal to microbes.10 All the other iodine types present contribute to staining and/or toxicity but have no antimicrobial activity.
The new patented super iodines are now available as oral rinses, irrigants, and gels. They contain as much as 100 times greater concentration of I2 than is available in PVP-I. At the same time, these new formulations contain only a few hundred ppm of the other nonbioactive iodine species, not the 31,600 ppm of nonbioactive species present in PVP-I. This dramatically increases efficacy while drastically minimizing overall toxicity. These cutting edge I2 formulations are not darkly colored and do not stain, even at 100 times the strength of PVP-I (figure 1).
In comparing the efficacy of PVP-I to CHX against six periodontal bacteria (Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, Tannerella forsythensis, Prevotella intermedia, and Streptococcus anginosus), Nakagawa et al. determined that PVP-I reduced all strains to below detectable limits within 15 seconds. By contrast, CHX treatment was less effective, allowing more than 1,000 viable colonies to remain after 60 seconds.11
ChloraPrep is a leading preoperative surgical skin prep containing two germicidal agents, 2% CHX (which is 17 times stronger than 0.12% CHX oral rinse) and 70% isopropyl alcohol. When compared to a 300 ppm I2 formulation (one of the new super iodines), it took ChloraPrep four times longer to inactivate a highly resilient fungus, Aspergillus brasiliensis, than did the I2 solution alone.4,9 PVP-I required five minutes to completely inactivate Staphylococcus aureus compared to a super iodine formulation that completely inactivated the same pathogen in one tenth of that time.9
Several studies have demonstrated the ability of iodine to inactivate Streptococcus mutans and to inhibit caries.12,13 A 25 ppm I2 mouth rinse completely inactivated rhinovirus in 30 seconds, whereas mouth rinses from some leading oral care companies were not effective at all.14 An I2 preprocedural mouth rinse for patients is a far better choice to inactivate aerosolized bacteria and viruses than currently available mouth rinses. A recent study conducted at the Tokyo Dental School Department of Periodontology concluded that at a 30-second contact time, iodine was effective in inactivating the bacteria in periodontal biofilms.15 A joint study conducted in three leading European dental schools found that the persistent effect (substantivity) of iodine on subgingival microbial flora was far greater than CHX persistence (31 days versus 7 days).16 I2 is rapidly effective against viruses, bacteria, fungi, and spores, and unlike CHX, does not permit the development of bacterial resistance.7I2 is not only safe for human administration, it is necessary for human health.17 Iodine is both natural and organic. It is sourced from naturally occurring minerals or harvested from seaweed, where it is abundantly present. Indeed, governments around the world mandate the use of iodized salt as an inexpensive public health measure to help ensure adequate dietary intake of iodine.
This new generation of molecular iodine oral-care products was evaluated in a pilot clinical study conducted by a board-certified periodontist. The study included 53 patients who used molecular iodine products daily as part of a routine home-care regime over a six-month period. Their effectiveness in reducing gingivitis and in slowing the progression of periodontal disease was noted along with this conclusion: “These products will likely be as therapeutically effective as scaling and root planing in the treatment and management of periodontal disease” (written communication, June 2015).
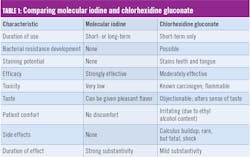
Two versions of molecular iodine oral-care products were recently evaluated by the independent Gordon J. Christensen Clinicians Report in a protocol that involved 50 dental offices and 250 patients. These were a ready-to-use oral rinse and a subgingival irrigant for use in an oral irrigating device. As a result of these evaluations, both products earned the distinction of inclusion in Clinicians Report Buying Guide: The Best Products for 2019 (for the official reprint, contact ioTech International).18 See Table 1 for a comparison of key properties of both molecular iodine and chlorhexidine gluconate oral rinses.
In every clinically important parameter, molecular iodine oral rinse outperforms CHX and does it without the side effects associated with CHX. There is an alternative to CHX—and it’s a better one.References
1. Balagopal S, Arjunkumar R. Chlorhexidine: the gold standard antiplaque agent. J Pharm Sci Res. 2013;5(12):270-274.
2. Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg. 2017;44:260-268. doi:10.1016/j.ijsu.2017.06.073.
3. Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217-4224.
4. BD. ChloraPrep with Tint Summary of Product Characteristics. Updated January 14, 2016.
5. Kampf G. Acquired resistance to chlorhexidine - is it time to establish an ‘antiseptic stewardship’ initiative? J Hosp Infect. 2016;94(3):213-227. doi:10.1016/j.jhin.2016.08.018.
6. Prasanna SGV, Lakshmanan R. Characteristics, uses, and side effects of chlorhexidine—a review. J Dent Med Sci. 2016;15(6):57-59.
7. Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res. 2002;37(5):389-398.
8. Kolsky RE, Moskowitz H, Kessler J, inventors. IoTech International, assignee. Stable compositions of uncomplexed iodine and methods of use. US patent 10,092,006. October 8, 2015.
9. Microbiological evaluation. Tokyo, Japan: Yoshida Pharmaceutical Co; April 2017.
10. Wada H, Nojima Y, Ogawa S, et al. Relationship between virucidal efficacy and free iodine concentration of povidone-iodine in buffer solution. Biocontrol Sci. 2016;21(1):21-27. doi:10.4265/bio.21.21.
11. Nakagawa T, Hosaka Y, Ishihara K, et al. The efficacy of povidone-iodine products against periodontopathic bacteria. Dermatology. 2006;212(Suppl 1):109-111.
12. Simratvir M, Singh N, Chopra S, Thomas AM. Efficacy of 10% povidone iodine in children affected with early childhood caries: an in vivo study. J Clin Pediatr Dent. 2010;34(3):233-238.
13. Herdiyati Y, Riyanti E, Prastuti D, Andisetyanto P. Stop caries with povidone iodine. Int J Sci Res. 2015;4(5):342-345.
14. Microbiological evaluation. Bozeman, Montana: BioScience Laboratories; 2014.
15. Hosaka Y, Saito A, Maeda R, et al. Antibacterial activity of povidone-iodine against an artificial biofilm of Porphyromonas gingivalis and Fusobacterium nucleatum. Arch Oral Biol. 2012;57(4):364-368. doi:10.1016/j.archoralbio.2011.09.005.
16. von Ohler C, Weiger R, Decker E, Schlagenhauf U, Brecx M. The efficacy of a single pocket irrigation on subgingival microbial vitality. Clin Oral Investig. 1998;2(2):84-90.
17. Iodine Fact Sheet for Health Professionals. National Institutes of Health website. https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/. Updated September 26, 2018.
18. CR Buying Guide: The Best Products for 2019. Clinicians Report. 2018;11(12):6.