Dental abscesses and their systemic effects
I recently read a blog post comparing silent abscesses to diseases like glaucoma, high blood pressure, and early coronary disease. What the author meant was that abscesses are also silent invasions and don’t warn us of the danger to our bodies. I thought the analogy was quite descriptive. A stack of research demonstrates that quiet infections can increase the chances of events such as heart attacks, strokes, and brain abscess due to the bacteria and their toxins causing inflammation inside the bloodstream.1,2 Whether silent or loud, when left alone, these infections can lead to osteomyelitis, cavernous sinus thrombosis, cellulitis, brain abscess formation, fasciitis of the neck or chest wall, and potentially sepsis. Even if those tragic events don’t occur, the infiltration of pathogenic bacteria and bacterial products activate the inflammatory response, which can compromise health.
Definitions and types of abscesses
By definition, a dental abscess is the final product of an inflammatory process, a suppurative collection associated with the structure surrounding the teeth caused by a bacterial infection. Acute dental abscesses are primarily periapical, periodontal, or pericoronal; other less common types include gingival or a combined periodontal-endodontic abscess.
Abscesses are polymicrobial, comprising various facultative anaerobes most commonly of the viridans group streptococci and the Streptococcus anginosus group along with strict anaerobes, especially anaerobic cocci Prevotella and Fusobacterium species.3 As technology advances, thanks to 16S rRNA gene sequencing and PCR (polymerase chain reaction) molecular techniques, we are able to identify more difficult to culture organisms such as Treponema, Atopobium, Bulleidia extructa, and Mogibacterium species, as well as Cryptobacterium curtum.3
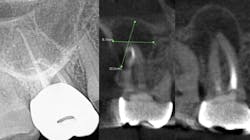
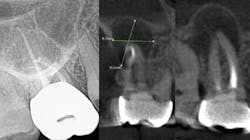
The periapical abscess that originates in the dental pulp is most often a secondary effect of dental caries. A periodontal abscess is an infection located contiguous to the periodontal pocket and may result in destruction of the periodontal ligament and alveolar bone. A periodontal abscess, the third most frequent dental emergency, has two ideologies: periodontitis related and nonperiodontitis related. Abscesses of periodontitis usually appear as an exacerbation of untreated periodontal disease or perhaps during periodontal treatment. Abscesses of nonperiodontitis-related origin frequently develop after the impaction of foreign objects, such as a piece of dental floss or abnormalities in the root enamel. A pericoronal abscess is associated with the crown of a partially erupted tooth. Like a gingival abscess, there may have been a retention of microbial plaque, food impaction, or trauma. The endo-periodontal lesions involve both the pulp and periodontal tissues, are relatively rare in clinical practice, and are one of the most challenging problems clinicians face (figure 1).
Periodontal abscesses
Etiology of periodontal abscesses can be from preexisting periodontal pockets or in healthy sites. In periodontal patients, subgroups of etiology can occur after nonsurgical therapy when dislodged calculus is pushed into the tissues or from inadequate scaling.4,5 Another occurrence can be after surgical therapy with the presence of foreign bodies, such as sutures or exacerbation of untreated periodontitis, refractory, or as described above in supportive periodontal therapy.4,6 What we often don’t think about is the use of systemic antimicrobials without subgingival debridement, which can cause abscess formation likely related to an overgrowth of opportunistic bacteria.7,8 In healthy sites, abscesses could be caused by trapped dental floss, a piece of a toothpick, a cracked tooth, or a perforation by an endodontic instrument.
The microbiology of periodontal abscesses is very similar to periodontal disease, predominately gram-negative anaerobic bacteria. The most prevalent bacterial species in periodontal abscesses is Porphyromonas gingivalis, ranging anywhere from 50% to 100%.9 Strict anaerobes detected include Prevotella intermedia, Prevotella melaninogenica, Fusobacterium nucleatum, Tannerella forsythia, Treponema spp. Parvimonas micra, Actinomyces spp., and Bifidobacterium spp., as well as facultative anaerobic gram-negative bacteria Campylobacter spp., Capnocytophaga spp., and Aggregatibacter actinomycetemcomitans.10
There are significant systemic ramifications with periodontal abscesses, whether due to a spread during therapy or being left untreated. During the treatment of an abscess, the concomitant bacteremia may lead to a colonization of pathogenic microorganisms to other body sites, such as pulmonary actinomycosis, brain abscess, or total knee arthroplasty infection.11-13 There are also reports of untreated abscesses that have led to cellulitis in breast cancer patients, cervical necrotizing fasciitis, or a sickle cell crisis in a patient with sickle cell anemia.14-16
Periapical abscesses
A periapical abscess, one of the most common dental emergencies, occurs at the apex of the tooth resulting from a bacterial infection of the pulp secondary to caries, tooth fracture, or even prior dental work. Pulpal infection can spread to a neighboring tooth from the infected periodontium or through the lateral canals. Signs and symptoms are like those of periodontal abscesses.
Acute abscess may manifest with swelling, sensitivity to hot and cold, biting, or chewing. A chronic periapical abscess often has less pain due to an intermittent draining through a sinus tract. The infection will taste foul and is often swallowed. A pathogen such as Porphyromonas gingivalis is resistant to stomach acid and linked to an imbalance in the gut’s microbiome, causing yet another area of dysbiosis. The pathogen Fusobacterium nucleatum can also make its way through the stomach biome and is overabundant in colorectal cancer.17 It isn’t just in the mouth, bacteria is on the move.
These abscesses may cause a fever or malaise as well as other systemic manifestations of an infection, including spread of the infection to the soft tissue (e.g., facial cellulitis or Ludwig’s angina, which is an infection of the floor of the mouth under the tongue) or the mandible/maxilla (osteomyelitis of the jaw). Infection may also result in sinusitis, cavernous sinus thrombosis, sepsis (blood infection), brain abscess, endocarditis, pneumonia, and potentially death.
The risk of spreading infection is amplified in the presence of a weakened immune system. Research released recently found of a group of 87 patients with brain abscesses, 52 with no primary source of infection had significantly higher counts of Streptococcus anginosus bacteria in their samples.18 S. anginosus, common bacteria of oral abscesses, can also lead to pharyngitis, bacteremia, and infections in other internal organs such as the lung and liver. Many hospital admissions have roots in dental sepsis.19,20
Cardiovascular complications and endodontic infections aren’t new news. In 2013, Pessi et al. found bacterial DNA, mainly oral viridans streptococci, at 78.2% in clots of patients who were in the throes of a heart attack.21 If left untreated, mortality rates can increase to 40% if the patient develops mediastinitis from a descending infection.22
More than 500 bacterial species have been detected in endodontic infections with the core microbiome as a select group of 20–30 species.22 They include several gram-negative bacteria (Fusobacterium nucleatum, Dialister species, Porphyromonas endodontalis, Porphyromonas gingivalis, Prevotella species, Tannerella forsythia, and Treponema species) and gram-positive (Parvimonas micra, Filifactor alocis, Pseudoramibacter alactolyticus, Olsenella uli, Actinomyces species, Streptococcus species, Propionibacterium species, and Cutibacterium acnes).23
Antibiotic therapy
Several antibiotics have been supported through the years, including penicillin, metronidazole, tetracyclines, and clindamycin. With antibiotic therapy, a rule of thumb is to consider susceptibility and resistance of the bacterial strain, patient allergies, and drug interactions. The ADA published a systematic review and made clinical recommendations for the “urgent management of symptomatic irreversible pulpitis with or without symptomatic apical periodontitis, pulp necrosis, and symptomatic apical periodontitis, or pulp necrosis and localized acute apical abscess using antibiotics, either alone or as adjuncts to definitive, conservative dental treatment (DCDT) in immunocompetent adults.”24 The ADA wrote: “Evidence suggests that antibiotics for the target conditions may provide negligible benefits and probably contribute to large harms.” They suggested that antibiotics for target conditions be used only when systemic involvement is present, and that immediate DCDT should be prioritized in all cases. Antibiotic use is recommended in patients with systemic involvement, “for example malaise or fever.”
I have complete understanding and regard for antibiotic stewardship, but how does one decide when an infection becomes technically systemic? A tooth infection that has spread is a medical emergency and can happen quickly, becoming pervasive and severe. Systemic infections can be life-threatening, and I would not want to be the gatekeeper for whether to prescribe or not prescribe.
While I am not an expert, I do know that a bacterial infection inside or outside the mouth isn’t stellar for the entire body. In more than 30 years of clinical care, I can’t count the number of times I’ve seen a radiolucent area on a radiograph, when asymptomatic the treatment was nothing. There were other cases where the patient was in pain, given an antibiotic, and sent on their way. They didn’t have a follow-up appointment to treat the infection, perhaps because they couldn’t afford it or didn’t want treatment. I’m assigning no blame here, but simply stressing that we need to continue to educate our patients. Pathogens circulate and sometimes require the body’s immune system to be on overdrive, and many people’s health can’t afford to have an overactive immune system. Technology is expanding and dentistry can now utilize cone beam imaging to find abscesses that don’t present on a 2D radiograph. We can help our patients become healthier. But a silent abscess is an abscess. My friends in a Nashville dental practice recently had a case where the periapical radiograph looked completely fine, yet when a cone beam was done, an abscess showed up louder than Loretta Lynn at the Grand Ole Opry. We should be stewards of new knowledge, new technology, and be open to the expanding world of science.
Editor’s note: This article appeared in the July 2023 print edition of RDH magazine. Dental hygienists in North America are eligible for a complimentary print subscription. Sign up here.
References
- Liljestrand JM, Mäntylä P, Paju S, et al. Association of endodontic lesions with coronary artery disease. J Dent Res. 2016; 95(12):1358-1365. doi:10.1177/0022034516660509
- Fu Y, Maaβ S, du Teil Espina M, et al. Connections between exoproteome heterogeneity and virulence in the oral pathogen Aggregatibacter actinomycetemcomitans. mSystems. 2022;7(3):e0025422. doi:10.1128/msystems.00254-22
- Robertson D, Smith AJ. The microbiology of the acute dental abscess. J Med Microbiol. 2009;58(Pt 2):155-162. doi:10.1099/jmm.0.003517-0
- Dello Russo NM. The post-prophylaxis periodontal abscess: etiology and treatment. Int J Periodontics Restorative Dent. 1985;5(1):28-37.
- Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK. Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities.J Periodontol. 1996;67(2):93-102. doi:10.1902/jop.1996.67.2.93
- Garrett S, Polson AM, Stoller NH, et al. Comparison of a bioabsorbable GTR barrier to a non-absorbable barrier in treating human class II furcation defects. A multi-center parallel design randomized single-blind trial. J Periodontol. 1997;68(7):667-675. doi:10.1902/jop.1997.68.7.667
- Helovuo H, Hakkarainen K, Paunio K. Changes in the prevalence of subgingival enteric rods, staphylococci and yeasts after treatment with penicillin and erythromycin. Oral Microbiol Immunol. 1993;8(2):75-79. doi:10.1111/j.1399-302x.1993.tb00548.x
- Topoll HH, Lange DE, Müller RF. Multiple periodontal abscesses after systemic antibiotic therapy. J Clin Periodontol. 1990;17(4):268-272. doi:10.1111/j.1600-051x.1990.tb00024.x
- Herrera D, Alonso B, de Arriba L, Santa Cruz I, Serrano C, Sanz M. Acute periodontal lesions. Periodontol 2000. 2014;65(1):149-177. doi:10.1111/prd.12022
- Hafström CA, Wikström MB, Renvert SN, Dahlén GG. Effect of treatment on some periodontopathogens and their antibody levels in periodontal abscesses. J Periodontol. 1994; 65(11):1022-1028. doi:10.1902/jop.1994.65.11.1022
- Suzuki JB, Delisle AL. Pulmonary actinomycosis of periodontal origin. J Periodontol. 1984;55(10):581-584. doi:10.1902/jop.1984.55.10.581
- Franceschi D, Giuliani V, Giuntini V, Pini Prato G. Brain abscess and periodontal pathogens (Fusobacterium nucleatum). Report of a case. Clin Case Rep. 2020;8(12):2488-2493. doi:10.1002/ccr3.3173
- Waldman BJ, Mont MA, Hungerford DS. Total knee arthroplasty infections associated with dental procedures. Clin Orthop Relat Res. 1997;(343):164-172.
- Manian FA. Cellulitis associated with an oral source of infection in breast cancer patients: report of two cases. Scand J Infect Dis. 1997;29(4):421-422. doi:10.3109/00365549709011842
- Chan CH, McGurk M. Cervical necrotising fasciitis--a rare complication of periodontal disease. Br Dent J. 1997;183(8):293-296. doi:10.1038/sj.bdj.4809498
- Rada RE, Bronny AT, Hasiakos PS. Sickle cell crisis precipitated by periodontal infection: report of two cases. J Am Dent Assoc. 1987;114(6):799-801. doi:10.14219/jada.archive.1987.0173
- Alon-Maimon T, Mandelboim O, Bachrach G. Fusobacterium nucleatum and cancer. Periodontol 2000. 2022;89(1):166-180. doi:10.1111/prd.12426
- Roy H, Bescos R, McColl E, et al. Oral microbes and the formation of cerebral abscesses: a single-centre retrospective study. J Dent. 2023;128:104366. doi:10.1016/j.jdent.2022.104366
- Wang J, Ahani A, Pogrel MA. A five-year retrospective study of odontogenic maxillofacial infections in a large urban public hospital. Int J Oral Maxillofac Surg. 2005;34(6):646-649. doi:10.1016/j.ijom.2005.03.001
- Thomas SJ, Atkinson C, Hughes C, Revington P, Ness AR. Is there an epidemic of admissions for surgical treatment of dental abscesses in the UK?. BMJ. 2008;336(7655):1219-1220. doi:10.1136/bmj.39549.605602.BE
- Pessi T, Karhunen V, Karjalainen PP, et al. Bacterial signatures in thrombus aspirates of patients with myocardial infarction. Circulation. 2013;127(11):1219-1228,e1-e6. doi:10.1161/CIRCULATIONAHA.112.001254
- Shweta, Prakash SK. Dental abscess: a microbiological review. Dent Res J (Isfahan). 2013;10(5):585-591.
- Siqueira JF Jr, Rôças IN. Present status and future directions: microbiology of endodontic infections. Int Endod J. 2022;55(Suppl 3):512-530. doi:10.1111/iej.13677
- Lockhart PB, Tampi MP, Abt E, et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: a report from the American Dental Association. J Am Dent Assoc. 2019;150(11):906-921.e12. doi:10.1016/j.adaj.2019.08.020
About the Author

Anne O. Rice, BS, RDH, CDP, FAAOSH
Anne O. Rice, BS, RDH, CDP, FAAOSH, founded Oral Systemic Seminars after over 35 years of clinical practice and is passionate about educating the community on modifiable risk factors for dementia and their relationship to dentistry. She is a certified dementia practitioner, a longevity specialist, a fellow with AAOSH, and has consulted for Weill Cornell Alzheimer’s Prevention Clinic, FAU, and Atria Institute. Reach out to Anne at anneorice.com.