The flu season
By Charles John Palenik
The most recent disease to emerge globally is severe acute respiratory syndrome (SARS). Notoriety and media attention surrounding the disease developed instantly. In the end, however, only 8,437 cases and 813 deaths were reported worldwide. There were 418 reported cases and no deaths in the Untied States. In mid-July, the World Health Organization declared the SARS epidemic over, at least for now.
What will not likely be front-page news is the upcoming influenza (flu) season. Yet influenza causes epidemics of severe illness and life-threatening complications almost every winter. Flu epidemics affect 10 to 20 percent of the population and are associated with an average of 36,000 deaths and 114,000 hospitalizations in the United States. More than 90 percent of deaths are people 65 years and older. However, more than 50 percent of infections requiring a hospital stay involve persons under the age of 65. The economic impact of a severe epidemic in the United States could approach $15 billion.
The virus that causes influenza was first isolated in 1933. There are three types of influenza viruses — type A, B, and C. Types A and B have been linked to human illnesses throughout the world. Type A is more common and is usually associated with more serious epidemics and pandemics. Type B causes more regional outbreaks, while type C results in mild respiratory illness and is not involved with epidemics.
Flu viruses are enveloped and covered with rigid projectiles called spikes. There are two basic types of spikes — hemagglutinin (HA) and neurominidase (NA). HA molecules allow the flu virus to adhere to host epithelial cells and then to penetrate. Its name comes from the ability of HA to agglutinate red blood cells in tissue cultures. NA molecules facilitate the release and spread of newly formed viral particles by preventing them from adhering immediately to host cells and each other. NA also helps flu viruses penetrate the mucous layers of the pharynx. The result is enhanced viral spread.
Human type A influenza viruses have subtypes identified through the use of a nomenclature system that includes the geographic site of discovery, specific laboratory identifier, year of discovery, and in parentheses, the HA and NA types present. An example would be A/Hong Kong/156/97(H5N1). Influenza types B and C do not have subtypes.
Flu viruses are constantly changing with the emergence of new strains and variants. Each year, one or two subtypes of type A and one of type B may be circulating in an area.
HA is the main antigen associated with immunity with NA playing a lesser role. Small changes in HA composition (antigenic drift) produce variant type A viruses that can infect partially immune people. Major changes in HA and NA (antigenic shift) produce type A distinct from those that have been circulating. The result could be populations that have no immunity against the new viral forms.
Influenza is transmitted via aerosolized or droplet transmission from the respiratory tract of an infected person. A less important mode of transmission is by direct contact with contaminated items.
Maximum communicability occurs one to two days before the onset of symptoms through four to five days thereafter. Flu season runs from November through April with a peak of activity from late December through late March.
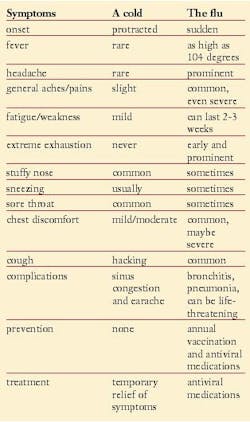
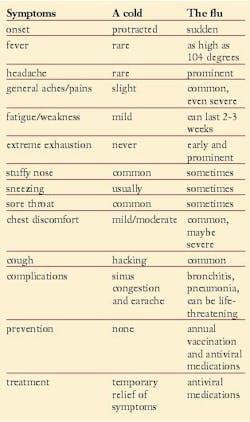
Unlike many other types of viral respiratory infections such as the common cold, influenza can have very serious outcomes. The signs and symptoms of the flu can be confused with other infections, especially colds (rhinoviruses). Also, colds and the flu commonly develop at the same time of year.
The best way to avoid the flu is by receiving an annual vaccination. Influenza vaccines available in the United States are composed of inactivated virus. Only disrupted or split-virus vaccines are used and are prepared using organic solvents or detergents. Split-virus vaccines are generally associated with fewer adverse events among children. The vaccine does not contain any viable virus and thus is incapable of causing infection. The composition of the trivalent 2003-2004 influenza vaccine in the United States is A/New Caledonia/20/99-like (H1N1), A/Moscow/10/99-like (H3N2), and B/Hong Kong/330/ 01-like viruses.
Vaccination of adults requires one intermuscular injection. Children under age nine require two injections. The vaccine is inexpensive, well tolerated, and has an overall protection rate of 70 to 80 percent. The risk that the vaccine will cause serious harm is extremely small. Problems are usually mild (soreness, redness or swelling at the injection site, fever or body aches) and only last a day or two.
On June 17, 2003, the FDA approved an intranasal, trivalent, cold-adapted, live, attenuated influenza vaccine called FluMist. It's designed to immunize healthy children and adolescents between the ages five and 17 and healthy adults ages 18 to 49. People nine years and older require a single dose, while children between five and eight years (who have not been previously vaccinated with FluMist) require two doses administered 60 days apart.
Efficacy varies by its similarity to the strain of influenza presently circulating in the population, the age of the recipient, and the presence of underlying illness. The vaccine has successfully matched circulating viral strains 11 out of the last 12 years.
Some people should not get a flu shot until they consult their primary health care provider. These include people with severe allergies to hen eggs, severe reactions to a flu shot in the past, or who developed Guillain-Barré syndrome during the six weeks after getting a flu shot.
An annual flu shot is recommended for the following groups considered to be at increased risk for serious complications.
• People who are 50 years of age or older
• Residents of long-term care facilities that house people with chronic medical conditions
• People older than six months who have long-term health problems (for example, heart or lung conditions, including asthma, diabetes, anemia, and kidney disease)
• People with weakened immune systems, including those caused by HIV/AIDS or medication
• Children and teenagers (ages six months to 18 years) who are on long-term aspirin therapy and therefore could develop Reyes Syndrome after the flu
• Women more than three months pregnant during the flu season.
Anyone can get influenza and readily give it to others. Vaccination is recommended for health care workers and others coming in close contact with people at risk of serious influenza.
The ideal time to receive a flu shot is in October or November. In the past when vaccine supplies were late in arriving, October vaccination was reserved for those at highest risk and health care workers.
However, this year's projected production and distribution schedules will allow for sufficient supply in both October and November. Everyone is encouraged to be immunized when the vaccine becomes available in their area.
Like other health care workers, dental hygienists should be vaccinated against influenza during October. Protection develops in two weeks and lasts about a year. Different viral strains may be present by that time. Successful vaccination prevents disease through occupational acquisition and limits spread to patients, co-workers, and family members.
The Organization for Safety and Asepsis Procedures (OSAP) is the leading source of safety and health information for dental practices. OSAP offers 22 links to valuable information on influenza at www.osap.org/general /search/index.php.
Charles John Palenik, MS, PhD, is an assistant director of Infection Control Research and Services at the Indiana University School of Dentistry. Dr. Palenik has authored numerous articles, book chapters and monographs, and is the co-author of the popular Infection Control and Management of Hazardous Materials for the Dental Team. He serves on the Executive Board of OSAP, dentistry's resource for infection control and safety.Questions about this article or any infection control issue may be directed to [email protected].