Selecting local anesthetic agents for nonsurgical periodontal therapy
A wide variety of dental local anesthetic agents that can be used to provide safe and effective local anesthesia for our patients is available to us. There is no one magic agent that works best for every situation. So how do we choose? Often dental hygienists select what they used in school, or their choices are limited by their supervising dentists, who may have only one or two agents available that they are accustomed to using.
Selection should be based on the individual needs of each patient: the length of procedure; patient profile, including medical contraindications; the need for hemostasis; and the need for posttreatment pain management.1–3 Nonsurgical periodontal therapy (NSPT) procedures are generally elective procedures requiring an intermediate-duration (approximately 60 minutes) category of anesthetics (table 1) and rarely require posttreatment pain management related to agent selection. Highlights on agent selection with regard to patient profile and need for hemostasis are incorporated in the discussions below, with focus on intermediate-duration agents (generic names) available in the United States and their general use for adult patients during NSPT.Agents and vasoconstrictors
The injectable dental local anesthetics available in the United States today are all amide local anesthetics. They are safe, nonallergenic, usually metabolized in the liver, and excreted by the kidneys (exceptions discussed later).
These agents are also mild vasodilators that increase the rate of anesthetic absorption into the bloodstream. The increased absorption rate leads to an increased risk of systemic toxicity, reduction of the duration of action, and increased bleeding in the area. Vasoconstrictors are added to local anesthetic agents to counteract the vasodilatory properties. By constricting the blood vessels in the area, the rate of absorption is decreased, which results in reduced risk of systemic toxicity, increased depth and duration of action, and increased hemostasis.
When providing local anesthesia, agents containing vasoconstrictors should be used unless there is an important reason not to or there exists an absolute contraindication to their use.2,4 An absolute contraindication describes a circumstance when a drug should not be administered under any circumstances because it is unsafe to do so. A relative contraindication describes a circumstance when the drug may be used carefully after thoughtful consideration of risk versus benefit and when a safer alternative is not available.1,2 Most of the patients we treat fall into the latter category because there are few absolute contraindications to the administration of dental local anesthetic agents with vasoconstrictor for patients whose health profiles, and American Society of Anesthesiologists (ASA) Physical Status Classifications, indicate that they are eligible for elective procedures such as NSPT (table 2).2,5
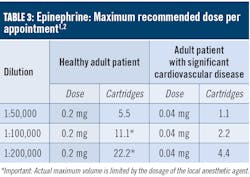
As discussed above, there are few absolute contraindications for the use of vasoconstrictors for patients presenting with a health profile eligible for NSPT. For patients taking tricyclic antidepressants, levonordefrin should be avoided, whereas an epinephrine dose and concentration should be reduced. In most situations, limiting the amount of vasoconstrictor a patient receives produces an adequate benefit without compromising patient safety. For example, a patient whose profile suggests a relative contraindication for vasoconstrictors (e.g., controlled hyperthyroidism) may often receive the lowest possible dose of epinephrine, not to exceed the 0.04 mg/appointment as recommended for patients with significant cardiovascular disease (table 3). The risks of using a vasoconstrictor versus the benefits should always be considered. Recall that inadequate pain and anxiety control may result in the release of unpredictable amounts of endogenous epinephrine and norepinephrine, perhaps exceeding the safe dose that would be provided by the clinician. Also consider that the more medically compromised a patient is, the greater the need for profound anesthesia will be to avoid exacerbation of symptoms.1,4
All dental local anesthetic cartridges with vasoconstrictors contain an antioxidant, usually sodium (meta) bisulfite. Bisulfites are also commonly found in food and beverages. Hypersensitivity to bisulfites has been reported, especially with steroid-dependent asthmatics, and reactions vary from mild to severe.8 Patients who present with a true documented bisulfite allergy should not receive a local anesthesia agent that contains a vasoconstrictor. Note that sulfite sensitivity should not be confused with sulfates or with sulfur drugs as there is no cross-allergenicity.4,8
Specific agents
Lidocaine HCl
Federal Drug Administration (FDA)–approved in 1948, lidocaine was the first amide dental local anesthetic and a great improvement over the ester agents previously available due to its more rapid onset, higher potency, more profound anesthesia, and longer duration of action. But perhaps its most important asset is that, as an amide, it is virtually nonallergenic. It remains the gold standard by which all others are judged and holds 49% of the US market share.4 In the United States, it is compounded with epinephrine as 2% lidocaine, 1:100,000 epinephrine, and 2% lidocaine, 1:50,000 epinephrine.
The lidocaine is absolutely contraindicated for patients with a true documented allergy to amide-type local anesthetics (extremely unlikely), and since it is formulated with epinephrine in the US, it is absolutely contraindicated for patients with known bisulfite allergy.
Mepivacaine HCl
FDA-approved in 1960, mepivacaine is available in the United States as 2% mepivacaine with 1:20,000 levonordefrin, and since 1961, also formulated as 3% mepivacaine plain (without vasoconstrictor). Mepivacaine has a milder vasodilatory effect than most other amides, and so the 3% plain formulation may be useful with patients for whom vasoconstrictor is contraindicated and cannot receive 4% prilocaine plain. However, 3% mepivacaine plain provides less profound anesthesia, and the duration of action is “short” (only 20 minutes administered as a supraperiosteal injection and 40 minutes administered as a nerve block), which makes it less desirable for procedures such as NSPT, which often require intermediate-duration agents as well as profound pulpal anesthesia. The 2% mepivacaine with levonordefrin formula provides similar onset and duration of action as lidocaine 1:100,000 epinephrine (table 1), but, as discussed above, the vasoconstrictor levonordefrin does not provide the hemostasis required for most NSPT procedures.
Mepivacaine is absolutely contraindicated in patients with true allergy to amide-type local anesthetics (extremely unlikely). The mepivacaine formulated with 1:20,000 levonordefrin is absolutely contraindicated for patients with known bisulfite allergy and should be avoided in patients taking tricyclic antidepressants.
Prilocaine HCl
FDA-approved in 1965, prilocaine is less toxic, has a milder vasodilatory effect than the other amides, and provides a slightly longer duration of action than the other intermediate duration agents. It is available as 4% prilocaine with 1:200,000 epinephrine and as 4% prilocaine plain. Both formulations are recommended for patients with epinephrine sensitivity and requiring intermediate duration of action.
An interesting feature regarding prilocaine plain is that when providing a block injection, it is the only intermediate-duration plain local anesthetic (table 1). It can be a good choice for patients for whom vasoconstrictor is contraindicated. Prilocaine is initially metabolized in the lungs and kidneys, and therefore metabolized more easily by the liver than lidocaine or mepivacaine, so it may also be a good choice for patients with hepatic disease.
With high doses (i.e., those greater than the maximum recommended dose) prilocaine has been shown to lower the blood’s oxygen-carrying capacity and is, therefore, relatively contraindicated for use with patients at risk for methemoglobinemia and patients with problems of oxygenation, such as sickle cell anemia, and cardiac/respiratory failure. It is also relatively contraindicated for patients who are receiving acetaminophen or phenacetin, as these drugs increase methemoglobin levels.
Prilocaine is absolutely contraindicated for patients with true allergy to amide-type local anesthetics (extremely unlikely) and, if using the 1:200,000 epinephrine formulation, for patients with known bisulfite allergy.
Articaine HCl
Articaine has been available in Europe since 1976, but was not marketed in the United States until 2000. According to Stanley Malamed, DDS, it is the first and only local anesthetic designed specifically for dentistry.4 It is the leading dental local anesthetic in Canada and Europe, and is now the second most popular dental local anesthetic in the United States, holding 39.3% of the US market share.2
The increasing popularity of articaine has been attributed to higher injection success rates due to increased lipid solubility, which results in faster diffusion through hard and soft tissues, including palatal root anesthesia with buccal maxillary injections and mandibular anesthesia with supraperiosteal injections.9 Studies have shown that articaine provided by infiltration can be very successful in the mandible, in the anterior region, when provided both buccally and lingually.10 When supplementing an inferior alveolar nerve block (IANB) with a buccal infiltration at the mandibular first molar, the anesthesia success rate of the IANB is greatly improved.11,12 Some studies have also shown that articaine provides a shorter onset and longer duration of action than lidocaine.13,14
Classified as an amide with amide and ester characteristics, it is 1.5 times more potent than lidocaine and has similar toxicity. In the United States, it is available as 4% articaine with 1:100,000 epinephrine and 4% articaine with 1:200,000 epinephrine. Biotransformation occurs primarily in the plasma but also in the liver. Because of its unique composition and biotransformation pathway, the elimination half-life (i.e., the time required for 50% of a drug to be removed from the blood) of articaine, as reported by manufacturers, is only 44 minutes,15 which is more than twice as fast as all other dental amide agents (table 4). Other experts report an even shorter 27-minute elimination half-life for articaine.1,2,4 In addition to listing the rates of elimination half-life for dental local anesthetics, table 4 also indicates the time it takes for a 98.5% reduction (six elimination half-lives) in the blood level.
Articaine’s short half-life results in a decreased risk of system toxicity, which is significant because articaine may be the local anesthetic of choice for patients for whom a higher rate of elimination from the blood is desired (e.g., medically compromised, pregnant, nursing, liver-disease).16 Few studies exist regarding the use of local anesthetics with pregnant or lactating women. A general approach regarding safe drugs taken by lactating mothers is to avoid nursing for the required number of hours based on the drug’s half-life. Since it has not yet been determined if articaine is excreted in breast milk, to ensure minimal exposure, the FDA suggests that after receiving articaine, nursing mothers “pump and dump” breast milk for approximately four hours (six elimination half-lives) before resuming breastfeeding. This would translate to nine hours (six elimination half-lives) of “pump and dump” for all other local anesthetics.2–4
Articaine is absolutely contraindicated for patients with true allergy to amide-type local anesthetics (extremely unlikely), and since it is formulated with epinephrine in the United States, it is absolutely contraindicated for patients with known bisulfite allergy.
Other considerations
There has been some controversy about the use of 4% local anesthetics such as prilocaine and articaine with regard to increased neurotoxicity and increased risk of paresthesia. Recommendations had included avoiding block anesthesia with articaine. Those early reports were anecdotal (nonclinical) and retrospective in nature, often reliant upon malpractice reports, and therefore lacked clinical controls and were often susceptible to reporting bias.16,17 Only one study published in the Journal of the California Dental Association was clinical in nature—“based on patients actually seen and examined by a single clinician.”17 Pogrel determined that the number of cases of paresthesia from articaine and lidocaine were proportional to their market share, and that the number of cases from prilocaine was somewhat disproportional to its market share.17
Experts have suggested that adverse effects for new medications are reported more frequently than for older medications (known as the “Weber effect”), and that the subsequent publicity/hysteria causes additional reporting, all of which wane after the middle to the end of the second year of a drug’s introduction, even as its usage increases.16
Recent in-vitro studies of human neuroblastoma cells exposed to local anesthetics, including articaine, lidocaine, mepivacaine, and prilocaine, demonstrated that articaine was the least neurotoxic of the dental amide local anesthetics, and that articaine exhibited the most favorable safety profile.18,19
Other experts assert that the IANB paresthesia is most likely related to mechanical trauma, not chemical trauma, because the lingual nerve is stretched out and is in the path of the sharp dental needle during provision of the IANB. This opinion is supported, in part, by the following:
- More than 90% of dental paresthesia cases reported occur in the mandible (70%-92% involve the lingual nerve).4
- Reports of paresthesia after provision of the Gow-Gates and Vazirani-Akinosi injections (where the lingual nerve is not in the path of needle advancement) are extremely rare.20
- There are no reports of paresthesia after the use of articaine in medicine.4
So how do we choose? Each of us, individually, must make decisions. We choose local anesthetic agents based upon our professional judgment, experiences, treatment planned, and the patient’s health profile. Although local anesthetics are very safe at therapeutic doses, we must always consider existing conditions and medications that may precipitate adverse effects. Then, after thoughtful consideration of the risks versus the benefits, we can make the appropriate decisions regarding agent selection. Information regarding the dosages, safety, and effectiveness of agents is constantly being updated. Over the years there have been many conflicting reports. It is therefore essential for us to continuously learn by exploring the current scientifically based literature, with careful consideration and evaluation of quality and reproducible clinical/in-vitro studies, so we may ensure the effective and safe provision of local anesthesia for our patients.
Editor's note: Originally posted in 2020 and updated regularly
References
- Logothetis DD. Local Anesthesia for the Dental Hygienist. 2nd ed. St. Louis, MO: Elsevier; 2017.
- Malamed SF. Handbook of Local Anesthesia. 7th ed. St. Louis, MO: Elsevier; 2019.
- Bowen DM, Pieren JA. Darby and Walsh Dental Hygiene. 5th ed. St. Louis, MO: Elsevier; 2020.
- Malamed SF. A Renaissance in Local Anesthesia. Presented at: International Seminars; December 1, 2018; San Jose, CA.
- ASA Physical Status Classification System. American Society of Anesthesiologists website. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Accessed October 2, 2019.
- Moore PA, Doll B, Delie RA, et al. Hemostatic and anesthetic efficacy of 4% articaine HCl with 1:200,000 epinephrine and 4% articaine HCl with 1:100,000 epinephrine when administered intraorally for periodontal surgery. J Periodontol. 2007;78(2):247-253. doi:10.1902/jop.2007.060314.
- Santos CF, Modena KC, Giglio FP, et al. Epinephrine concentration (1:100,000 or 1:200,000) does not affect the clinical efficacy of 4% articaine for lower third molar removal: a double-blind, randomized, crossover study. J Oral Maxillofac Surg. 2007;65(12):2445-2452. doi:10.1016/j.joms.2007.04.020.
- Sulfite sensitivity. The Asthma & Allergy Center website. https://www.asthmaandallergycenter.com/article/sulfite-sensitivity/. Accessed October 10, 2019.
- Katyal V. The efficacy and safety of articaine versus lignocaine in dental treatments: a meta-analysis. J Dent. 2010;38(4):307-317. doi:10.1016/j.jdent.2009.12.003.
- Meechan JG. The use of the mandibular infiltration anesthetic technique in adults. J Am Dent Assoc. 2011;142 Suppl 3:19S-24S. doi:10.14219/jada.archive.2011.0343.
- Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J. 2009;42(3):238-246. doi:10.1111/j.1365-2591.2008.01507.x.
- Haase A, Reader A, Nusstein J, Beck M, Drum M. Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block. J Am Dent Assoc. 2008;139(9):1228-1235. doi:10.14219/jada.archive.2008.0338.
- Costa CG, Tortamano IP, Rocha RG, Francischone CE, Tortamano N. Onset and duration periods of articaine and lidocaine on maxillary infiltration. Quintessence Int. 2005;36(3):197-201.
- Batista da Silva C, Berto LA, Volpato MC, et al. Anesthetic efficacy of articaine and lidocaine for incisive/mental nerve block. J Endod. 2010;36(3):438-441. doi:10.1016/j.joen.2009.12.014.
- Septocaine prescribing information. Septodont website. https://www.septodontusa.com/sites/default/files/2016-03/SeptocainePI.pdf. Accessed September 13, 2019.
- Malamed S. Articaine 30 years later. https://www.oralhealthgroup.com/features/1003919408/. Published February 4, 2016.
- Pogrel MA. Permanent nerve damage from inferior alveolar nerve blocks: a current update. J Calif Dent Assoc. 2012;40(10):795-797.
- Malet A, Faure MO, Deletage N, Pereira B, Haas J, Lambert G. The comparative cytotoxic effects of different local anesthetics on a human neuroblastoma cell line. Anesth Analg. 2015;120(3):589-596. doi:10.1213/ANE.0000000000000562.
- Albalawi F, Lim JC, DiRenzo KV, Hersh EV, Mitchell CH. Effects of lidocaine and articaine on neuronal survival and recovery. Anesth Prog. 2018;65(2):82-88. doi:10.2344/anpr-65-02-02.
- Malamed SF. A Renaissance in Local Anesthesia. Presented at: International Seminars; March 19, 2016; San Francisco, CA.
About the Author

Laura J. Webb, MS, RDH, FAADH
Laura J. Webb, MS, RDH, FAADH, is an experienced clinician, educator, and speaker who founded LJW Education Services (ljweduserv.com). She has provided educational methodology courses and accreditation consulting services for allied dental education programs and CE courses for clinicians. Laura has frequently spoken on the topics of local anesthesia and nonsurgical periodontal instrumentation. She was the recipient of the 2012 ADHA Alfred C. Fones Award. Laura can be reached at [email protected].
Updated May 2023