Using smart technology to outsmart patient challenges
Premier's Enamelon gel offers a home-care solution to, well, just about everyone in your operatory
BY ANNE NUGENT GUIGNON, RDH, MPH
The first cell phones were big, clunky, weighed a lot, and had a very short battery life. Very few people had them, but I distinctly recall a patient who had his own plumbing business. He insisted on carrying his phone everywhere. He embraced the future even though this now-primitive device only allowed him to make and receive phone calls.
Most everyone you know has some kind of mobile device - a smartphone, a tablet, or even a little flip phone. People have learned to rely on technology to solve problems and to simplify their lives. My iPhone does not have a single note of music on it, except for the blues ringtone, but it is indispensable, functioning as a phone, calendar, alarm, email and texting device, and allows me to keep connected with my husband and colleagues as I travel around the country.
My 90-year-old mother just announced she was getting a new iPhone and recently bought her first iPad. With the exception of phone calls, she uses her devices in very different ways. The parents of my teenage nieces and nephews consider smartphones an important safety tool, a way to communicate with their children immediately, and the kids' smartphones are used in ways I've never considered.
So what do smart devices have to do with clinical dental hygiene care? Actually, everything! People's lives are crazy today, and most are looking for ways to be smart and employ smart technology to simplify their lives - a lesson we can take into the treatment room. Patients expect us to deliver outstanding advice and treatment that promote optimal oral health, so we need to be smart and keep their lives as simple as possible.
Who is at risk for caries, erosion, periodontal disease, and hypersensitivity?
Nearly every patient we treat is at risk for some form of dental disease. The risk factors for the classic conditions - caries, gingivitis, and periodontal disease - are well known. While many patients feel that oral disease is just a matter of time, dental professionals need to be on the front line identifying and monitoring risk factors that lead to disease.
Over the last decade, the caries management by risk assessment (CAMBRA) movement has cast a big spotlight on effectively identifying patients at risk for caries, demineralization, and erosion.1 CAMBRA goals include developing effective protocols to either minimize disease risks or provide effective, targeted treatment protocols. This includes evaluation of salivary flow rates and composition, recognizing lifestyles, dietary intakes and behaviors that increase disease risk, and comorbid medical conditions.
-----------------------------------------------
Other articles by Guignon
- Dental germaphobics: When patients are not 'crazy'
- On Cheaters: Loupes are designed for specific vision shortcomings
- Growing a professional backbone
-----------------------------------------------
Hard tooth structures exposed over and over, for protracted periods of time to pH levels in the acidic range are at risk for both caries and hypersensitivity.2 Enamel begins to demineralize when the pH falls below 5, while exposed roots begin to deteriorate at pH 6.3 Much attention is now being paid to xerostomia, or hyposalivation, and its role in caries, demineralization, erosion, and hypersensitivity.4 The risk for these dental diseases skyrocket when the salivary flow rate is reduced to the point where the oral cavity is no longer in homeostasis.
Dry mouth favors the growth of both acidogenic and aciduric microbes - bacteria that produce copious amounts of acid or are acid tolerant.5 Acidic conditions also promote vigorous growth of Candida albicans, an aggressive yeast organism that is implicated in early childhood caries and many other oral conditions.7 C. albicans favors acidic environments and is a heavy acid producer.8
Erosion, classified as the loss of hard tooth structure that does not involve bacterial activity, is considered the leading cause of dentinal hypersensitivity.3,4,9 In addition, research has shown that patients with hypersensitivity have eight times more open dentinal tubules that are twice the mean diameter than those who do not have this problem.10 Dentinal hypersensitivity, fueled by dietary factors and the increase in xerostomia, is growing in all age cohorts. Young children to seniors are at increasing risk for developing hypersensitivity.11-16
Developing a dry mouth
Lack of sufficient moisture in the oral cavity alters the taste of food, causes food to stick to teeth, increases the adhesion of plaque biofilm on tooth surfaces, leads to sore oral mucosa, and interferes with normal speech patterns.2,12,13
Dry mouth is the most common side effect for both prescribed and over-the-counter medications. A wide range of medications can mediate or potentiate dry mouth issues. Medications used to control or modulate hypertension, anxiety, depression, pain, seasonal allergies, asthma, appetite control, and nausea can each contribute to xerostomia.15
Contemporary lifestyles also play a role. Dehydration can occur from insufficient fluid intake or prolonged exposure to low levels of humidity. Emotional conditions such as stress and anxiety can impact salivary flow. Cigarette smoking and recreational drugs such as methamphetamine and cannabis or laxative abuse cause dry mouth. Caffeine and alcoholic beverages are diuretics, and foods high in sodium, such as soft drinks, salty snacks, and highly processed foods add to the problem.17
Most mouth rinses contain large amounts of alcohol, a necessary ingredient to keep therapeutic ingredients active, but alcohol can exacerbate oral dryness for those at risk. Even seemingly innocuous activities such as strenuous exercise,18 wearing a CPAP machine, or prolonged speaking can contribute to dry mouth.17 Oral appliances such as partial dentures, sports mouthguards, removable orthodontic aligners, occlusal guards, and whitening trays create a physical barrier to saliva, creating a temporary dry area.
Typically, natural salivary flow decreases with age,12,13,15 but dry mouth is now a well-recognized complication for a number of medical conditions including autoimmune disorders,19,20 radiation treatment to the head and neck area, salivary gland dysfunction, chronic renal failure, HIV/AIDS, Parkinson's disease, and diabetes.21 Xerostomia is also an issue for those who experience nasal blockage, sleep apnea, chronic obstructive pulmonary disease,22 gastric esophageal reflux disease,23 hormonal imbalances, and decreased estrogen levels created by either natural or surgically-induced menopause.
Seniors are at the highest risk for dry mouth24 for two different reasons. They are more likely to have one or more diseases or disorders that cause dry mouth and they typically take more medications. It is estimated that those over the age of 60 take three medications daily on average, increasing the overall risk for xerostomia.17
Soft drink mania and the health crazies
Study after study has demonstrated that modern drinks have fueled the erosion explosion.25-31 Erosive drinks are impossible to avoid, and the general public has been led to believe that energy drinks and sports drinks promote health and well-being, even though the pH of these drinks is typically in the 2.5 to 4.5 range.30-32 When sales of carbonated beverages began to decline a decade ago, marketers created whole new classifications of beverages and beverage enhancers. People not only drink traditional beverages; they now down gallons of new acidic drinks, including flavored waters, refrigerated teas and coffee, fruit-based teas, and water enhanced with flavored powders and flavor drops.
All of these drinks are loaded with acid. The primary culprit is citric acid, but that is only the beginning of the story. Most new drinks contain multiple organic acids that are used for flavoring and carbonation. Multiple acids increase titratable acidity, meaning it takes longer for the oral cavity to return to a neutral pH.1,29,30,32,33 Tooth structures exposed to these drinks are at risk for demineralization for longer periods of time, and the problem is further compounded for those with dry mouth who have insufficient salivary flow and reduced buffering capacity.2-4 One classic study looked at traditional Coke vs. Red Bull. Even though the pH of traditional Coke is 10 times lower than Red Bull, the titratable acidity of the energy drink is three times longer than the carbonated soft drink.34
The erosion story gets scarier. The pH of most brands of bottled water is 5, which has the potential to erode root structure over time. Add in sour acid candies, sugar-sweetened gummy vitamins, or calcium chews that are loaded with sugar, and the teeth are at even more risk.35 So-called "health food evangelists" frequently recommend drinking hot water laced with fresh lemon juice for weight loss or drinking apple cider vinegar to improve digestion.36
Reviewing the clinical day
Pretend you are reviewing the day's schedule, making notes as you go through the charts. Your morning schedule looks like this:
• 8 a.m. Megan - 49-year-old corporate attorney, training for her third Iron Man competition, drinks energy and sports drinks
• 9 a.m. Barrel-chested Bob - always full of great jokes, diabetic, bad gag reflex, and suffers from GERD and dentinal hypersensitivity, runs a pool cleaning service
• 10 a.m. Great Aunt Martha - early dementia, living in assisted care, rheumatoid arthritis in her hands, history of COPD
• 11 a.m. Little Johnny - a bright, fidgety 11-year-old, first visit since getting full orthodontic bands, Mom and Dad are high caries risk
• 11:30 a.m. Amy - Little Johnny's 24-month-old sister, second visit, thumb sucker, goes to sleep with a bottle every night, attends a private day care
It's going to be a busy day. Each patient has unique clinical challenges. Their disease risks are different, compliance issues are different, and their interest in health is different. Imagine having one home-care product that is formulated to help prevent caries, reduce the risk of gingivitis, promote remineralization, treat dentinal hypersensitivity, and help those with dry mouth. That may sound impossible; but just as today's smartphones are smarter than the original versions, Enamelon Preventive Treatment Gel is formulated with smart chemistry that can help solve a wide range of patient problems.
Using tried-and-true chemistries and new ingredients
Decades ago, there was an over-the-counter toothpaste called Enamelon. The science behind it was amazing, but the delivery system was challenging. So despite its promising benefits, it disappeared from the marketplace.
Today's Enamelon shares the same name as the original product, but it is not toothpaste. It took years to create the new formula. Enamelon Preventive Treatment Gel is a synergistic combination of active and inactive ingredients: stannous fluoride, ACP (amorphous calcium phosphate), spilanthes extract, Ultramulsion, and copolymers. It is an easy-to-use, brush-on gel designed to prevent and treat disease.
Stannous fluoride remineralizes and hardens enamel.37-40 It is a tried-and-true ingredient ideal for patients at risk for caries and those with dentinal hypersensitivity.41-42 ACP, also known as amorphous calcium phosphate, provides a rich source of calcium and phosphate, necessary building blocks for remineralization.43 For years, scientists tried combining calcium and phosphate with different types of fluoride with varying levels of success.
Both the natural tooth surface and fluoride ions are negatively charged, but adding calcium (CA) and phosphate (P) ions together carries a positive charge, which consequently facilitates the infusion of stannous fluoride into the tooth surface. To prevent premature reactivity, the calcium and phosphate ions in Enamelon are an anhydrous formula. Once the gel comes in contact with saliva the calcium and phosphate ions are released and are ready to form a bioavailable ACP compound to aid in remineralization.
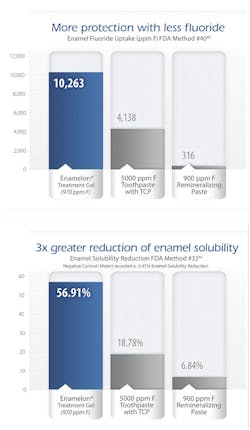
Enamelon Treatment Gel contains 970 PPM stabilized stannous fluoride and ACP, a fluoride ion level 80% less than prescription-strength 5,000 PPM treatment products. Fluoride-containing products that claim to either have significant fluoride uptake or reduce enamel solubility must be tested using the FDA protocols. Laboratory testing using the FDA Method #33 demonstrated Enamelon's proprietary SnF2/ACP formula reduces enamel solubility nearly three times more than prescription fluoride toothpastes and eight times more than a 900 PPM fluoride/calcium phosphate remineralizing paste.44 Tests using FDA Method #40 showed the fluoride uptake of Enamelon was more than double that of a 5,000 PPM fluoride toothpaste.45 Research is ongoing.
A stannous fluoride level lower than what is in over-the-counter toothpastes may seem counterintuitive. But when combined with calcium and phosphate, aided by specific copolymers, the magic of ACP is delivered to the oral cavity.44 The copolymers help improve substantivity, creating long-lasting salivary reservoirs that are rich in calcium and fluoride. Furthermore, the fluoride, calcium, and phosphate uptake at the tooth surface occludes the open dentinal tubules as well as strengthens enamel.45 The result of these actions reduces sensitivity and the risk of caries and erosion. In addition, there is increasing concern regarding excess fluoride ingestion and the potential to develop fluorosis as both primary and secondary teeth are developing.46-48
Unlike other fluoride formulations, stannous has both bactericidal and bacteriostatic effects suggesting that Enamelon can be an effective product for preventing and treating gingivitis. Stannous fluoride is also known to inhibit bacterial adhesion on tooth surfaces.49-51
Enamelon contains Ultramulsion and spilanthese extract, two ingredients that contribute to the uniqueness of this product. Ultramulsion, a saliva-soluble compound, moisturizes and lubricates soft tissues and aids in a pleasant mouth feel, a much-sought-after benefit for those who suffer from dry mouth. Spilanthes, a natural herb known since biblical times, stimulates salivary flow.
Gel treatment and final touches
Enamelon Preventive Treatment Gel should be used prior to bedtime, preferably after brushing with standard toothpaste. Since the gel formula does not contain abrasive particles, it does not damage exposed root structure or eroded areas. Ideally, patients should expectorate the excess without rinsing, allowing the chemistry to remain active and working at night.
Through the years, toothpaste advertisements have shown photos of toothbrushes loaded with thick ribbons of paste. Not only is this practice wasteful, there is also a potential to ingest considerable amounts of fluoride over time that could lead to fluorosis as primary and secondary teeth develop. In 2014, the American Dental Association Council on Scientific Affairs published new guidelines about the amount of toothpaste used by children. A pea-size amount is recommended for children from ages 3 to 6, while only a smear, which is a quarter size of a pea, is recommended for those under the age of three.46
It is virtually impossible to accurately dispense an appropriate amount for a child from traditional toothpaste tubes. The opening on the Enamelon tube is 3 mm in diameter as compared to a standard toothpaste tube with a 9 mm diameter opening. The smaller opening allows more precision control and eliminates dispensing excess product destined to go down the drain. Enamelon is gluten free, dye free, and does not contain SLS, a standard foaming agent found in many prescription and OTC products.
While stannous fluoride products can create surface stain, most stain is a direct result of dietary intake from beverages such as wine or coffee, coupled with inadequate biofilm removal. While Enamelon does not contain abrasives to remove stain; the Ultramulsion should limit surface stains. Brushing with a traditional OTC whitening paste is recommended to control any residual stain.
Back to the treatment room
Today's patients are incredibly complex and want simple solutions. Enamelon is formulated to meet multiple risk factors. As you learn more about the morning's patients, you'll see why Enamelon would be an appropriate choice for each situation:
• Megan is at a very high risk for dry mouth. Her exercise routine causes intermittent dry mouth challenges. She is experiencing estrogen loss due to perimenopause, and her job is high stress, both factors in daily dry mouth problems. While Jane drinks a lot of water, she also has a high intake of both energy and sports drinks and likes to drink wine with her evening meals. She also suffers from seasonal pollen allergies, treating these episodes with OTC antihistamines and nasal decongestants.
• Bob has a complex medical history. He was diagnosed with adult-onset diabetes three years ago and can't seem to get his A1C levels under control. Even though he would benefit from a three-month dental hygiene visit, he will only come when his insurance plan will cover the visit, even though he has been diagnosed with generalized moderately severe gingivitis. Bob suffers from GERD, uses a CPAP machine at night, and now complains of sensitivity when the ultrasonic scaler is used. Bob's home care is marginal, and he has a bad gag reflex, so brushing on the lingual of the lower molars is a real challenge.
• Great Aunt Martha's medications are a page-and-a-half-long computer printout. Even though she never smoked, she has developed COPD from years of being around secondhand smoke. Food does not taste right anymore, and it hurts to eat anything but highly processed foods. She has been losing weight, so the staff supplements her diet with Ensure dietary supplement. It's hard to find a staff member that really wants to help Martha brush her teeth or clean her partial denture, especially when she gets confused and is unwilling to cooperate.
• Johnny's parents and grandparents have a history of caries. Like most boys his age, he thinks his teeth are clean when the toothpaste foams up. Johnny just got full orthodontic bands, so his risk for gingivitis and decay just shot through the roof. He plays little league baseball and is on the soccer team and is encouraged to drink "sports drinks" for rehydration. Periodically, he uses an asthma inhaler.
• Amy just hit the terrible twos and is driving everyone in the house crazy at night, especially when her mother is trying to brush her teeth. Amy is a fussy eater so she gets a gummy bear vitamin every morning after breakfast. Even though her parents know better, they let her go to sleep with a bottle filled with milk. She drinks diluted fruit juice from a sippy cup at daycare and loves to snack on Goldfish crackers.
Enamelon Preventive Treatment Gel is a safe and effective nonprescription product available for dispensing only through the dental office. Each case contains 12 four-ounce tubes.
Today's patients are complicated. We're charged with delivering quality care and advice that focuses on individual needs and risk factors. Just like the smartphones that serve different needs for different users, we now have a smart treatment gel that can provide outstanding prevention and care for a wide variety of patient challenges. RDH
Disclosure: Supported by an educational grant from Premier.
ANNE NUGENT GUIGNON, RDH, MPH, provides popular programs, including topics on biofilms, power driven scaling, ergonomics, hypersensitivity, and remineralization. Recipient of the 2004 Mentor of the Year Award and the 2009 ADHA Irene Newman Award, Anne has practiced clinical dental hygiene in Houston since 1971.
References
1. Chaffee BW, Cheng J, Featherstone JD. Baseline caries risk assessment as a predictor of caries incidence. J Dent. 2015 Feb 27.
2. Mandel ID. The functions of saliva. J Dent Res. 1987 Feb;66 Spec No:623-7.
3. Lussi A, Schlueter N, et al. Dental erosion - an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011;45.
4. Zero DT, Lussi A. Erosion - chemical and biological factors of importance to the dental practitioner. Int Dent J. 2005;55(4 Suppl 1):285-90.
5. Almståhl Al, Wikström M. Oral microflora in subjects with reduced salivary secretion. J Dent Res. 1999 Aug;78(8):1410-6.
6. Takahashi Nl, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011 Mar;90(3):294-303.
7. Falsetta ML, Klein MI, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014 May;82(5):1968-81.
8. Almståhl A, Wikström M. Microflora in oral ecosystems in subjects with hyposalivation due to medicines or of unknown origin. Oral Health Prev Dent. 2005;3(2):67-76.
9. Eliasson L, Carlén A, et al. Dental plaque pH and micro-organisms during hyposalivation. J Dent Res. 2006 Apr;85(4):334-8.
10. Absi EG, Addy M, Adams D. Dentine hypersensitivity. The development and evaluation of a replica technique to study sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1989 Mar;16(3):190-5.
11. Trushkowsky RD, Garcia-Godoy F. Dentin hypersensitivity: differential diagnosis, tests, and etiology. Compend Contin Educ Dent. 2014 Feb;35(2):99-104; quiz 104.
12. Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010 Sep;55(3):238-44.
13. Flink H. Studies on the prevalence of reduced salivary flow rate in relation to general health and dental caries, and effect of iron supplementation. Swed Dent J Suppl. 2007;(192).
14. Thomson WM, Lawrence HP, et al. The impact of xerostomia on oral-health-related quality of life among younger adults. Health Qual Life Outcomes. 2006 Nov 8; 4:86.
15. Anil S, Vellappally S, et al. Xerostomia in geriatric patients: a burgeoning global concern. J Investig Clin Dent. 2014 Sep 1.
16. Amarasena N, Spencer J, et al. Dentine hypersensitivity in a private practice patient population in Australia. J Oral Rehabil. 2011 Jan; 38(1):52-60.
17. www.sjogrens.org/home/about-sjogrens-syndrome/symptoms. Accessed March 1, 2015.
18. Mulic A, Tveit AB, et al. Dental erosive wear and salivary flow rate in physically active young adults. BMC Oral Health. 2012 Mar 23;12:8.
19. Donaldson M, Epstein J, et al. Managing the care of patients with Sjögren syndrome and dry mouth: comorbidities, medication use and dental care considerations. J Am Dent Assoc. 2014 Dec;145(12):1240-7.
20. Olate S, Muñoz D, et al. A descriptive study of the oral status in subjects with Sjögren's syndrome. Int J Clin Exp Med. 2014 Apr 15;7(4):1140-4.
21. Noboru Kuroiwa D, Ruiz Da Cunha Melo MA, et al. Evaluation of salivary flow and drug interactions in patients with a diagnosis of diabetes mellitus. Minerva Stomatol. 2014 Nov-Dec;63(11-12):421-6.
22. Nordén J, Grönberg AM, et al. Nutrition impact symptoms and body composition in patients with COPD. Eur J Clin Nutr. 2015 Feb;69(2):256-61.
23. Corrêa MC, Lerco MM, et al. Salivary parameters and teeth erosions in patients with gastroesophageal reflux disease. Arq Gastroenterol. 2012 Jul-Sep;49(3):214-8.
24. Wiener RC, Wu B, et al. Hyposalivation and xerostomia in dentate older adults. J Am Dent Assoc. 2010 Mar;141(3):279-84.
25. Zero DT. Etiology of dental erosion - extrinsic factors. Eur J Oral Sci. 1996 Apr;104(2 ( Pt 2)):162-77.
26. Flink H. Studies on the prevalence of reduced salivary flow rate in relation to general health and dental caries, and effect of iron supplementation. Swed Dent J Suppl. 2007;(192):3-50.
27. Murrell S, Marshall TA, et al. Comparison of in vitro erosion potentials between beverages available in the United Kingdom and the United States. J Dent. 2010 Apr;38(4):284-9.
28. Jensdottir T, Holbrook P, et al. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006 Mar;85(3):226-30.
29. Brown CJ, Smith G, et al. The erosive potential of flavoured sparkling water drinks. Int J Paediatr Dent. 2007 Mar;17(2):86-91.
30. Cochrane NJ, Yuan Y, et al. Erosive potential of sports beverages. Aust Dent J. 2012 Sep;57(3)359-64.
31. Malinauskas BM, Aeby VG, et al. A survey of energy drink consumption patterns among college students. Nutr J. 2007 Oct 31;6:35.
32. Ehlen LA, Marshall TA, et al. Acidic beverages increase the risk of in vitro tooth erosion. Nutr Res. 2008 May;28(5):299-303.
33. Bartlett DW, Fares J, et al. The association of tooth wear, diet and dietary habits in adults aged 18-30 years old. J Dent. 2011 Dec;39(12):811-6.
34. Owens BM. The potential effects of pH and buffering capacity on dental erosion. Gen Dent. 2007 Nov-Dec;55(6):527-31.
35. Wagoner SN, Marshall TA, et al. In vitro enamel erosion associated with commercially available original-flavor and sour versions of candies. J Am Dent Assoc. 2009 Jul;140(7):906-13.
36. 20 reasons you should drink lemon water in the morning. http://complete-health-and-happiness.com/20-reasons-drink-lemon-water-morning/.
37. Rošin-Grget K, Peroš K, et al. The cariostatic mechanisms of fluoride. Acta Med Acad. 2013 Nov;42(2):179-88.
38. Makin SA. Stannous fluoride dentifrices. Am J Dent. 2013 Mar;26 Spec No A:3A-9A.
39.Faller RV, Eversole SL. Enamel protection from acid challenge - benefits of marketed fluoride dentifrices. J Clin Dent. 2013;24(1):25-30.
40. Aspiras M, Stoodley P, et al. Clinical implications of power toothbrushing on fluoride delivery: effects on biofilm plaque metabolism and physiology. Int J Dent. 2010;2010:651869.
41. Parkinson C, Hughes N, et al. The efficacy of an experimental dentifrice containing 0.454% w/w stannous fluoride in providing relief from the pain of dentin hypersensitivity: an 8-week clinical study. Am J Dent. 2013 Mar;26 Spec No A:25A-31A.
42. Burnett GR, Willson RJ, Lucas RA. In vitro studies investigating the dentin tubule-occlusion properties of an experimental anhydrous stannous fluoride dentifrice. Am J Dent. 2013 Mar;26 Spec No A:10A-14A.
43. Schemehorn BR, Orban JC, et al. Remineralization by fluoride enhanced with calcium and phosphate ingredients. J Clin Dent. 1999;1013-6.
44. Schemehorn BR, DiMarino JC, Movahed N. Comparison of the enamel solubility reduction from various prescription and OTC fluoride toothpastes and gels. J Clin Dent. 2014;25:61-4.
45. Schemehorn BR, DiMarino JC, Movahed N. Comparison of the incipient lesion enamel fluoride uptake from various prescription and OTC fluoride toothpastes and gels. J Clin Dent. 2014;25:57-60.
46. American Dental Association Council on Scientific Affairs. Fluoride toothpaste use for young children. J Am Dent Assoc. 2014 Feb;145(2):190-1.
47. Basch CH, Rajan S. Marketing strategies and warning labels on children's toothpaste. J Dent Hyg. 2014 Oct;88(5):316-9.
48. Basch CH, Hammond R, et al. Advertising of toothpaste in parenting magazines. J Community Health. 2013 Oct;38(5):911-4.
49. Boyd RL. Long-term evaluation of a SnF2 gel for control of gingivitis and decalcification in adolescent orthodontic patients. Int Dent J. 1994 Feb;44:119-30.
50. Mazza JE, Newman MG, et al. Clinical and antimicrobial effect of stannous fluoride on periodontitis. J Clin Perio. 1981;8: 203-212.
51. Hernández Guerrero JC, Acosta Torres S, et al. In-office study to evaluate the effects of a stabilized stannous fluoride dentifrice and an advanced manual toothbrush regimen on gingival health and plaque accumulation in Mexican adults. Am J Dent. 2014 Dec;27(6):296-300.