Nonsurgical attachment gain: A protocol for achieving periodontal reattachment
By Judy Carroll, RDH, and Howard M. Notgarnie, RDH, EdD
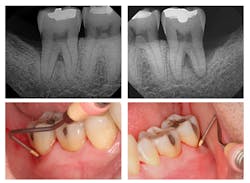
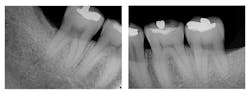
Figure 1: Pre-treatment radiographs
Chronic periodontitis is the most common form of periodontitis, and aggressive periodontitis causes rapid destruction of the supporting structures of the teeth.1 The chronic, inflammatory nature of periodontitis is a likely mechanism that makes it a risk factor for cardiovascular disease,2 preeclampsia,3 and cancers of the skin, breast, lung, esophagus, and gallbladder.4 From 2009 through 2012, periodontitis was present in 46% of US adults and severe periodontitis in 8.9% of US adults.5 Demographic characteristics showing an increased risk for periodontitis were male, nonwhite, poor, uneducated, and elderly.5 Treatment of periodontitis has been scaling and root planing (SRP), surgical intervention, and the use of chemotherapeutic and biologic agents.1
Traditional SRP followed by periodontal surgery can save teeth and regenerate periodontal structures supporting those teeth.6 Periodontal regeneration consists of two histologically identifiable improvements-cementum and connective-tissue growth-and two clinically identifiable improvements-bone growth and coronal migration of the epithelial attachment.7 Although SRP is often done in preparation for surgery,1 visually enhancing nonsurgical techniques with a periodontal endoscope might further improve outcomes while decreasing costs and morbidity when the surgical option can be avoided.
This case report documents a nonsurgical protocol to achieve reattachment of periodontal tissues that had been lost to periodontitis.
Case description
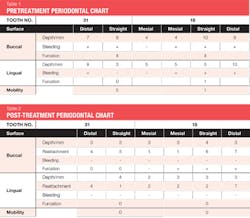
The patient was 45 years old with at least one known family member having periodontitis. He had a normal level of C-reactive protein (2.0 mg/L), slight elevation of blood pressure (146/87 mmHg), low-density lipoproteins (130 mg/dL), and slight deficiencies of vitamin D and thyroxin. He used an appliance to control bruxism and had a history of orthodontic treatment, although malocclusion was still present. A periodontist had recommended extraction of teeth Nos. 18 and 31, which had vertical defects on the distal surfaces (see Figure 1). On both teeth, recession was less than 1 mm on all surfaces, and pocket depths were at least 7 mm on the buccal and distal surfaces. Tooth No. 18 had 11.8 mm bone loss on the distal surface, a Class II buccal furcation with a 10 mm pocket, and a Class I lingual furcation with a 5 mm pocket. Tooth No. 31 had 8.4 mm bone loss on the distal surface and a Class II furcation with an 8 mm pocket on the buccal surface (see Table 1).
The dental hygiene diagnosis was localized, severe chronic periodontitis. Occlusal trauma was a contributing factor to the initiation of periodontitis; occlusal trauma and mucogingival deformity were contributing factors to disease progression.
Protocol-The patient took 20 mg doxycycline twice a day for 180 days beginning two weeks prior to clinical treatment. Dental hygiene care comprised SRP on teeth Nos. 18 and 31, with adjunctive therapy of enamel matrix derivative (EMD) under local anesthetic. A periodontal endoscope enhanced treatment site visualization during SRP, removal of granulomatous tissue, preparation of the root surfaces with ethylenediaminetetraacetic acid (EDTA), and application of EMD. The patient returned to his dental hygienist of record for periodontal maintenance.
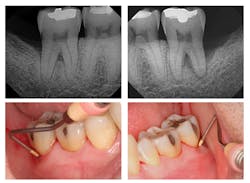
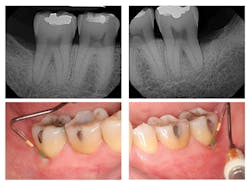
Evaluation-A six-month evaluation demonstrated reattachment (see Figure 2). The radiograph of tooth No. 18 exhibited 7.4 mm bone loss on the distal surface, reflecting an increased bone height of 4.4 mm. For tooth No. 31, the radiograph exhibited 6.2 mm bone loss, reflecting an increased bone height of 2.2 mm. Epithelial attachment gain was clinically evident as well (see Table 2). Pocket depths had marked improvement with elimination of furcation involvement on both teeth, decreased mobility of tooth No. 18, and no increase in recession. The patient also submitted three-year post-treatment radiographs taken by the dental hygienist of record (see Figure 3) with evidence of bone stability.
Periodontal Endoscopy
Bacteria in plaque, particularly Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, initiate a host immune response and promote a plaque biofilm resistant to antimicrobial agents.8 The host's response to bacterial pathogens associated with periodontitis can destroy periodontal tissues.8-10 This host response includes matrix metalloproteinases (MMPs) that destroy collagen, allowing leukocytes to enter the area and fight infection.11 Golub et al. demonstrated that tetracycline analogues reduce collagen destruction associated with MMP function.12 Subantimicrobial doses of doxycycline inhibit the destructive mechanisms of MMPs,11,13-15 thereby making it effective as an adjunct to SRP.8,10
Inflammation inhibits extracellular matrix formation and decreases the ability of stem cells from the periodontal ligament and dental follicle to differentiate into cementoblasts, osteoblasts, and fibroblasts.16 EMD is a biologic agent composed of proteins, primarily amelogenins, derived from pig tooth germs.17,18 EMD promotes stem cell differentiation and blast cell activity,18,19 thereby speeding bone growth.20,21
The effectiveness of EMD is unclear. Use of EMD as an adjunct to flap debridement did not improve radiographic bone growth compared with flap debridement alone22 or compared to the use of nanohydroxyapatite as an adjunct to periodontal surgery.23 However, EMD did reduce postsurgical recession24 and gene expression of inflammatory markers when occlusal forces were not excessive,25 and it enhanced effectiveness of nanohydroxyapatite as an adjunct to periodontal surgery.26
Evidence that nonsurgical application of EMD promotes periodontal regeneration has been equivocal. In one clinical study, EMD as an adjunct to SRP increased attachment primarily by long junctional epithelium rather than bone growth.27 In another study, researchers using EMD found no significant difference in pocket depth or gain of attachment compared with controls receiving only SRP.28 On the other hand, a nonsurgical approach on four single-rooted teeth generated histological and clinical evidence of periodontal regeneration, although the amount of regeneration was not sufficient to save those teeth.7
Periodontal endoscopy might play a role in the success of this protocol to achieve periodontal reattachment. The periodontal endoscope aids the clinician in gingival retraction and visualization of dental root surfaces.29,30 Studies have demonstrated efficacy of a periodontal endoscope as a tool for detection of calculus. A periodontal endoscope improved clinicians' ability to detect31 and remove32 calculus compared to tactile detection and instrumentation. After in vivo treatment using the periodontal endoscope and six months' healing, calculus was absent on 35 of subjects' 36 specimens examined in vitro after extraction.33 Growth of new bone and junctional epithelium was evident with lack of inflammation.33 Patients perceived less pain during periodontal endoscopy examination than by tactile examination with a probe,34 thus there is potential for improved compliance with treatment recommendations.
Figure 2: Six-month post-treatment radiographs and photos
Figure 3: Three-year post-treatment radiographs
Combining systemic host modulation and targeted administration of enamel matrix derivative with periodontal endoscopy was effective as a nonsurgical protocol to achieve attachment gain. Because evaluation in this case was clinical, we do not know if periodontal regeneration has occurred. Histological studies subsequent to this protocol could establish the effective presence or absence of true periodontal regeneration. RDH
Judy Carroll, RDH, is the founder and clinical director of PerioPeak Innovations. She has been a clinical practitioner for 26 years and teaches advanced endoscopic techniques and comprehensive integrative periodontal therapy using a multidisciplinary approach. Howard M. Notgarnie, RDH, EdD, is president-elect of the Colorado Dental Hygienists' Association. He has been practicing clinically for 24 years and has given presentations at local, national, and international dental hygiene meetings.
References
1. Gehrig JS, Willmann DE. Foundations of Periodontics for the Dental Hygienist. 4th ed. Philadelphia, PA: Wolters Kluwer; 2016.
2. Yooprasert P, Siribamrungwong M. Interrelationship between periodontitis and cardiovascular diseases. Int J Exp Dent Sci. 2013;2:110-117. doi:10.5005/jp-journals-10029-1051.
3. Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Relationship between periodontitis and preeclampsia: A meta-analysis. PLoS One. 2013;8:e071387. doi: 10.1371/journal.pone.0071387.
4. Nwizu NN. Periodontal Disease and Cancer: Chronic Inflammation-The Oral-Systemic Link (dissertation). Ann Arbor, MI: State University of New York at Buffalo; 2014.
5. Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009-2012. J Periodontol. 2015;86:611-622. doi:10.1902/jop.2015.140520.
6. Gupta S, Deshmukh J, Khatri R, Kulkarni VK, Karthik B. From hopeless to good prognosis: Journey of a failing tooth. J Int Oral Health. 2015;7:53-57.
7. Mellonig JT, Valderrama P, Gregory HG, Cochran DL. Clinical and histologic evaluation of nonsurgical periodontal therapy with enamel matrix derivative: A report of four cases. J Periodontol. 2009;80:1534-1540. doi:10.1902/jop.2009.090160.
8. Leszczynska A, Buczko P, Buczko W, Pietruska M. Periodontal pharmacotherapy-An updated review. Adv Med Sci. 2011;56:123-131. doi:10.2478/v10039-011-0044-9.
9. Gu Y, Walker C, Ryan ME, Payne JB, Golub LM. Non-antibacterial tetracycline formulations: Clinical applications in dentistry and medicine. J Oral Microbiol. 2012;4:19227. doi: 10.3402/jom.v4i0.19227.
10. Zia A, Bey A, Andrabi SM. Host modulation for management of periodontal diseases. Guident. 2014;7:50-52.
11. Honibald E, Mathew S, Padmanaban J, Sundaram E, Ramamoorthy R. Perioceutics: Matrix metalloproteinase inhibitors as an adjunctive therapy for inflammatory periodontal disease. J Pharm Bioallied Sci. 2012;4:417-421. doi:10.4103/0975-7406.100315.
12. Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, et al. Minocycline reduces gingival collagenolytic activity during diabetes: Preliminary observations and a proposed new mechanism of action. J Periodontal Res. 1983;18:516-526.
13. Moreno Villagrana AP, Gómez Clavel JF. Antimicrobial or subantimicrobial antibiotic therapy as an adjunct to the nonsurgical periodontal treatment: A meta-analysis. ISRN Dent. 2012;581207. doi:10.5402/2012/581207.
14. Farhad SZ, Aminzadeh A, Mafi M, Barekatain M, Naghney M, Ghafari MR. The effect of adjunctive low-dose doxycycline and licorice therapy on gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. Dent Res J. 2013;10:624-629.
15. Sadarangani SP, Estes LL, Steckelberg JM. Non-anti-infective effects of antimicrobials and their clinical applications: A review. Mayo Clin Proc. 2015; 90:109-127. doi:10.1016/j.mayocp.2014.09.006.
16. Liu J, Wang L, Liu W, Li Q, Jin Z, Jin Y. Dental follicle cells rescue the regenerative capacity of periodontal ligament stem cells in an inflammatory microenvironment. PLoS One. 2014;9:e108752. doi:10.1371/journal.pone.0108752.
17. Maycock J, Wood SR, Brookes SJ, Shore RC, Robinson C, Kirkham J. Characterization of a porcine amelogenin preparation, EMDOGAIN, a biological treatment for periodontal disease. Connect Tissue Res. 2002;43:472-476. doi:10.1080/03008200290000880.
18. Nazirkar G, Singh S, Dole V, Nikam A. Effortless effort in bone regeneration: A review. J Int Oral Health. 2014;6:120-124.
19. Miron RJ, Bosshardt DD, Zhang Y, Buser D, Sculean A. Gene array of primary human osteoblasts exposed to enamel matrix derivative in combination with a natural bone mineral. Clin Oral Investig. 2013;17:405-410. doi:10.1007/s00784-012-0742-0.
20. Miron RJ, Bosshardt DD, Gemperli AC, Dard M, Buser D, Gruber R, et al. In vitro characterization of a synthetic calcium phosphate bone graft on periodontal ligament cell and osteoblast behavior and its combination with an enamel matrix derivative. Clin Oral Investig. 2014;18:443-451. doi:10.1007/s00784-013-0977-4.
21. Miron RJ, Wei L, Bosshardt DD, Buser D, Sculean A, Zhang Y. Effects of enamel matrix proteins in combination with a bovine-derived natural bone mineral for the repair of bone defects. Clin Oral Investig. 2014;18:471-478. doi:10.1007/s00784-013-0992-5.
22. Ragghanti Zangrando MS, Chambrone D, Pasin IM, Conde MC, Pannuti CM, Pugliesi Alves de Lima LA. Two-year randomized clinical trial of enamel matrix derivative treated infrabony defects: Radiographicanalysis. BMC Oral Health. 2014;14:149. doi:10.1186/1472-6831-14-149.
23. Al Machot E, Hoffmann T, Lorenz K, Khalili I, Noack B. Clinical outcomes after treatment of periodontal intrabony defects with nanocrystalline hydroxyapatite (Ostim) or enamel matrix derivatives (Emdogain): A randomized controlled clinical trial. J Biomed Biotechnol. 2014;786353. doi:10.1155/2014/786353.
24. Yilmaz S, Kuru B, Altuna-Kiraç E. Enamel matrix proteins in the treatment of periodontal sites with horizontal type of bone loss. J Clin Periodontol. 2003;30:197-206. doi:10.1034/j.1600-051X.2003.10190.x.
25. Nokhbehsaim M, Deschner B, Winter J, Bourauel C, Jäger A, Jepsen S, et al. Anti-inflammatory effects of EMD in the presence of biomechanical loading and interleukin-1β in vitro. Clin Oral Investig. 2012;16:275-283. doi:10.1007/s00784-010-0505-8.
26. Pilloni A, Saccucci M, Di Carlo G, Zeza B, Ambrosca M, Paolantonio M, et al. Clinical evaluation of the regenerative potential of EMD and nanoHA in periodontal infrabony defects: A 2-year follow-up. Biomed Res Int. 2014;492725. doi:10.1155/2014/492725.
27. Sculean A, Windisch P, Keglevich T, Gera I. Histologic evaluation of human intrabony defects following non-surgical periodontal therapy with and without application of an enamel matrix protein derivative. J Periodontol. 2003;74:153-160. doi:10.1902/jop.2003.74.2.153.
28. Gutierez MA, Mellonig JT, Cochran DL. Evaluation of enamel matrix derivative as an adjunct to nonsurgical periodontal therapy. J Clin Periodontol. 2003;30:739-745. doi:10.1034/j.1600-051X.2003.00374.x.
29. Carroll J. The evolution of care. Modern Hygienist. 2006;2:46-48.
30. Kwan JY. Enhanced periodontal debridement with the use of micro ultrasonic, periodontal endoscopy. Oral Health. 2006;96:47-48,51-52,55-56,58-59.
31. Osborn J, Lenton P, Lunos SA, Blue C. Endoscopic vs. tactile evaluation of subgingival calculus. J Dent Hyg. 2014;88:229-236.
32. Geisinger ML, Mealey BL, Schoolfield J, Mellonig JT. The effectiveness of subgingival scaling and root planing: An evaluation of therapy with and without the use of the periodontal endoscope. J Periodontol. 2007;78:22-28. doi:10.1902/jop.2007.060186.
33. Wilson TG, Carnio J, Schenk R, Myers G. Absence of histologic signs of chronic inflammation following closed subgingival scaling and root planing using the dental endoscope: Human biopsies-a pilot study. J Periodontol. 2008;79:2036-2041. doi:10.1902/jop.2008.080190.
34. Poppe K, Blue C. Subjective pain perception during calculus detection with use of a periodontal endoscope. J Dent Hyg. 2014;88:114-123.