Cracking the sterilization packaging code
Paper, plastic, and a bit of adhesive … how difficult can that be to manage? As the mechanics and sophistication of sterilization technology have evolved through the years, sterilization packaging has become more complex, with a range of choices in materials, designs, and features. Cracking the code of sterilization packaging begins with understanding the method of sterilization to be used (e.g., saturated steam, dry heat, unsaturated chemical vapor), the type and design of packaging material, and the instruments to be sterilized. Then it’s on to selecting the best materials to wrap and secure the contents for the delivery of sterile instruments for use with patients.
Dentistry leans toward the choice between plastic or paper peel pouches when packaging loose instruments, and sterilized “blue” wrap when using an instrument organization system such as cassettes. This article will describe the differences in the most popular choices.
Medical device and standards
The US Food and Drug Administration (FDA) regulates sterilization packaging intended for use in health care under CFR Title 21 880.6850 and classifies it as a class II medical device.1 Manufacturers must submit a 510(k) outlining the validation testing and results in order to market products.
Intended use—A sterilization wrap (pack, sterilization wrapper, bag, or accessories) is a device intended to enclose another medical device that is to be sterilized by a health-care provider. It is intended to allow sterilization of the enclosed medical device and also to maintain sterility of the enclosed device until it is used.1
Based on the validation testing, the best practice is to follow the manufacturer’s instructions for use (IFU). Any alteration of the IFU for sterilization wrap is considered an off-label use of the product and could negatively impact the efficacy and safety of the product used during sterilization of instruments. Written policies and protocols for packaging techniques in each practice should be based on the product manufacturer’s written IFU to ensure safety of reusable patient care items. It should be noted that not all packaging systems are suitable for all types of sterilization methods; use of improper materials may prevent or reduce penetration of the sterilant.
Selecting effective packaging materials
- With a sterilization packaging system, consideration should be given to the following properties:2
- Compatibility between the method of sterilization and items to be sterilized
- Allows effective penetration of the sterilant and permits adequate air removal
- Ensures adequate barrier to microorganisms during processing, handling, and storage
- Maintains protection of contents and resists tearing or puncture
- Provides complete seal integrity and is tamper-evident
- Easy to use and allows for aseptic presentation
- Free of toxic ingredients and nonfast dyes
- Non linting
Types of sterilization packaging
While there is a wide range of packaging materials available, most dental practices choose single-use disposable, paper-plastic peel pouches or flat sheets of sterilization wrap.
It’s in the bag
Offered in various sizes, configurations, and types of built-in chemical indicators, sterilization pouches are used with loose groups of instruments and are typically compatible with steam and ethylene oxide (EtO) sterilization. The use of medical-grade Kraft paper and a transparent polypropylene/polyester laminate film (minimum 2 mm thick) is recommended. Be discerning when choosing a product, as some manufacturers use recycled instead of medical-grade paper. Medical Kraft paper used in sterilization pouches has a basic weight of 40 to 60 pounds and contains only approved additives, has controlled porosity, and has no pinholes greater than 0.5 mm.3 The steam enters and exits only through the paper side of the pouch and the pores must effectively close during the drying process to maintain sterility of the contents.
Selecting the appropriate pouch size prevents overloading of instruments and allows penetration of the sterilant with adequate air removal and less chance of puncture or tearing. There should be a minimum of one inch of space between the top of the instruments or cassette and the seal of the pouch.4 If a pouch is too large it will allow excessive movement of the instruments, which may result in tears or puncture. A pouch that is too small may cause tearing and prevent adequate steam penetration and air removal and may impact drying. Most dental practices benefit from having a variety of pouch sizes to meet their procedural needs.
After instruments have been positioned in the pouch, the pouch should be placed flat on the counter and the instruments should be in a single layer with at least one quarter of an inch of clearance on each side for package contraction and proper circulation.5 Instruments should not be stacked like a cord of firewood! As much air as possible should be pressed from the pouch prior to sealing with the adhesive strip to prevent the pouch from bulging during sterilization and potentially breaking the heat seals on the three heat-sealed sides. The fold lines on the flap, which are often perforated, should be strictly followed to prevent tunneling or overlapping and small openings between the package and the sealant adhesive. Additionally, the seal’s fold should be free of wrinkles or creases, which prevents closure integrity.
For an aseptic presentation, single-ended instruments, such as ultrasonic inserts, forceps, and dressing pliers, should be positioned in a pouch with the handle/grasp or nonworking end toward the thumb notch and away from the adhesive-sealed end. Hinged instruments should be placed and held in an “open” position.
Paper or plastic?
Best practice dictates placing pouches on their edges in a rack, like books on a shelf. This configuration allows for efficient 360-degree circulation and penetration of steam while also being conducive to the drying process. Pouches should not be touched until they are dry to reduce the chance of wicking and tearing of the paper. If it’s common practice in your office to place the pouches flat or horizontally on the shelf, the sterilizer manufacturer’s instructions for use must be followed in relation to plastic or paper side orientation. Note, the pouches must not overlap.
Wrap it up—I’ll take it!
Flat nonwrappers are made of spunbond-meltblown-spunbond (SMS) materials that are bonded together to form sheets and are available in a wide variety of sizes and weights. Wraps should be selected according to the size, shape, and weight of the device or cassette to be processed. The wrap should allow four to six inches around the four sides of the cassette to ensure complete coverage of the contents. The wrap should not be too large in order to prevent air pockets from forming, which can inhibit the penetration and release of the steam.
Typically, two layers of wrap are required per the manufacturer’s validated IFU; some manufacturers may offer two sheets ultrasonically sealed at the two edges. The two-layer concept provides aseptic protection of the inner layer wrap and sterile contents should the outer layer become torn, exposed to environmental elements (dust), or compromised. Additionally, the two layers allow for aseptic presentation for surgical settings by removal of the outer wrap.
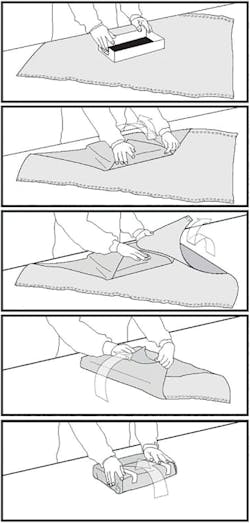
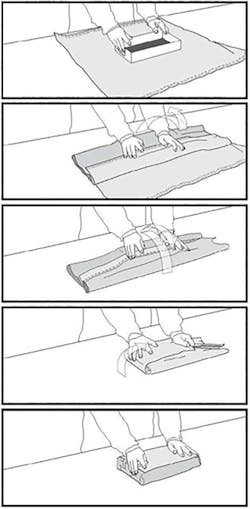
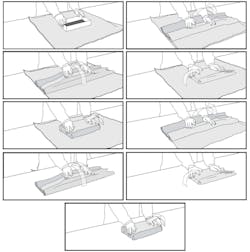
The two recommended wrapping methods are the envelope/diagonal fold and the package/square fold (figures 1 and 2). The diagonal fold is recommended for small- to medium-sized cassettes and the square fold is recommended for large cassettes. The correct technique when wrapping with flat wraps is to make sure the first fold completely covers the package all the way to the bottom of the instrument set, not just over the top of the set. The wrapping should be snug. If the wrapper is too loose, it can create water puddles that can result in wet packs or it can leave openings that may cause contamination. If the package is too tight, it could hinder the sterilant from entering and leaving the package.
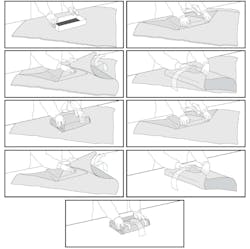
There is a choice of simultaneous (two layers at once) or sequential (gift within a gift concept), which is wrapping the item completely using one layer of wrap and then wrapping a second time with another layer of wrap (figures 3 and 4). Note that with the sequential method, no tape is placed to secure the last fold of the inside wrap. Instead, it is held securely in place with the application of the second layer of wrap.
For quality assurance, include a chemical indicator inside the cassette to verify that the steam reached the inside of the package. Because the wrap is opaque, an external chemical indicator must also be used.
The plastic laminate used in pouches is impervious to steam and might prevent the sterilant from reaching the surface of anything with which the plastic side is in physical contact. Therefore, pouches should not be used within wrapped cassettes.
Package labeling
Proper labeling of sterilization packaging allows for identification of contents, date tracking for purposes of quality assurance, inventory control, stock rotation, and instrument recall. Nontoxic permanent soft marking pens that are approved for the sterilization process (ASTM D4236) should be used to write on packages. It is critical that writing be done only on the plastic laminate of pouches or on the sterilization tape or affixed label with wrapped cassettes. The paper should never be written on, as this may compromise the integrity.
At a minimum, sterile packs are to be labeled with the sterilizer used, the cycle or load number, the date of sterilization, and an expiration date if applicable.6
Chemical indicators
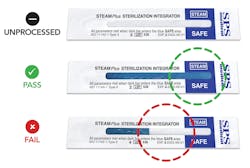
The International Organization for Standardization (ISO) standards for performance make it clear that the only way to be sure an item has been effectively steam-sterilized is to expose it to all three sterilization variables—time, temperature, and the presence of steam. Yet many traditional sterilization pouches have simply an external Type 1 process indicator validating only exposure to heat (temperature). Advanced technology is available with pouches that have both internal and external multiparameter indicators, which confirms all three criteria for sterilization have been achieved and the contents have been exposed to the critical variables. Remember that chemical indicators do not contain microbial spores, therefore, they cannot prove that the contents of the pouch are sterile.7 The use of this multiparameter pouch in every load—combined with a Type 5 integrating indicator at least once a day (preferably every load) and weekly biological monitoring—provides a high level of sterility assurance.
It’s a date!
Based on sound scientific principles, the shelf life of a properly processed pouch or pack is event-related, not time-related, and dependent upon proper handling and storage. The shelf life of a packaged sterile item depends on the quality of the packaging material, the storage conditions, and the amount of handling.2 A sterile product will remain sterile as long as the packaging is not compromised. Those conditions include not being torn, wet, soiled, or physically damaged.
It is important to note that all sterilization packaging and accessories have a manufacturer’s materials expiration date and may list a related shelf-life date. For example, a pouch may have a three-year from date of manufacture expiration date and a two-year expiration from date of sterilization. This information is critical to stock rotation. Use of sterilization packaging after expiration dates is considered off-label use by the FDA.
In closing
Instrument reprocessing and sterilization are hidden from patient view. Be proactive and share with your patients the standardized protocol conducted in your office to ensure patient safety during treatment. Armed with the knowledge of the criteria for selection and use, the code for sterility assurance is demystified … because you can’t see sterile.
References
1. US Food & Drug Administration Code of Federal Regulations Title 21, Volume 8, 21CFR880.6850. Revised April 1, 2019.
2. Comprehensive guide to steam sterilization and sterility assurance in healthcare facilities. ANSI/AAMI ST79: 2017. The Association for the Advancement of Medical Instrumentation website. https://my.aami.org/store/detail.aspx?id=ST79.
3. Nagaraja PA. Hospital Sterilization. Chapter 6. New Dehli; Jaypee Brothers Medical Publishers; 2011.
4. Central Service Technical Manual .7th ed. Dallas, Texas. International Association of Healthcare Central Service Material Management; 2007:111-316.
5. Seavey R. Packaging systems for sterilization. Nursing Lesson Plan 618. IAHCSMM website. https://www.iahcsmm.org/images/Lesson_Plans/Nursing_Plans/Nursing618.pdf. Published November/December 2016. Accessed December 27, 2019.
6. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Centers for Disease Control and Prevention website. https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care2.pdf. Published October 2016. 12-13;32-34.
7. Miller CH. Infection Control and Management of Hazardous Materials for the Dental Team. 5th ed. St. Louis: Mosby Elsevier; 2014:120-151.
With more than 20 years of academic and directorial experience, LEANN KEEFER, MSM, RDH, has a reputation as a trailblazer and is an international speaker in infection prevention. In her role as director of clinical services and education for Crosstex International, Keefer identifies trends in health care and infection prevention, developing and implementing the company’s strategies relating to education and professional relations. Keefer was appointed to the Organization for Safety, Asepsis and Prevention (OSAP) Association Board of Directors in 2012 and serves on various foundation and publication boards in the US and Canada.
About the Author

Leann Keefer, MSM, RDH
With more than 20 years of academic and directorial experience, LEANN KEEFER, MSM, RDH, has a reputation as a trailblazer and is an international speaker in infection prevention. In her role as director of clinical services and education for Crosstex International, Keefer identifies trends in health care and infection prevention, developing and implementing the company’s strategies relating to education and professional relations. Keefer was appointed to the OSAP Association Board of Directors in 2012 and serves on various foundation and publication boards in the US and Canada.