Instrument Sterilization in Dentistry
by Eve Cuny, RDA, MS; Helene Bednarsh, RDH, MPH
Educational objectives
Upon completion of this course, the dental healthcare professional (DHCP) will be able to:
1. Follow an appropriate process for the segregation, cleaning and sterilization of re-usable items, and for the appropriate disposal of single-use items.
2. Understand the various cleaning and sterilization devices available in the United States, as well as the various methods by which they sterilize and clean instruments.
3. Select the correct materials used in preparing instruments prior to sterilization, package instruments appropriately for sterilization and ensure correct storage of sterile instruments.
4. Understand the various tests that are available to ensure the sterility of instruments, and what these tests can be used for and will show.
Abstract
Effective and efficient infection control in the dental office is essential for the safety of patients and to ensure that productivity does not suffer. Infection control programs all include the cleaning and sterilization of reusable dental instruments and devices. Care must be taken by the dental healthcare professional to ensure that all instruments are cleaned prior to sterilization, and that this is carried out in a safe manner to avoid injury and puncture wounds. Use of closed-system cassettes reduces the risk to dental healthcare professionals when executing infection control programs. When using ultrasonic cleaners, washers and sterilizers, it is important to always follow the manufacturer’s instructions. It is also important to consult with the manufacturer of dental instruments and devices as needed to ensure complete sterilization and to avoid damage to these items. Assurance of sterility of instruments and devices can be obtained through the use of one of several tests, and these tests must be performed regularly to ensure that the sterilizer is sterilizing all instruments and devices and that these are safe for use on patients.
Introduction
Today’s busy dental practices face a serious challenge: to maintain or increase productivity while ensuring that patient safety remains a top priority. At times, these may seem like incompatible goals. Advances in dental processing equipment, however, have empowered practices to develop safer processes while realizing efficiencies and ultimately, saving money.
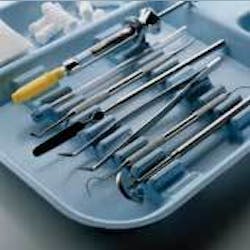
A cleaning and sterilization process that meets ADA and CDC guidelines is vital to an effective infection control program.1 Streamlining of this process requires an understanding of proper methods, materials, and devices. Many methods of instrument reprocessing are available. Use of a complete system that encompasses and fulfills all elements that are critical maximizes efficiency and minimizes risks. Closed cassette systems provide a more efficient and safer way to process, sterilize and organize instruments in a dental office (Figure 1) - these eliminate manual steps during instrument reprocessing such as hand scrubbing and time-consuming sorting of instruments, thereby improving safety and increasing efficiency.
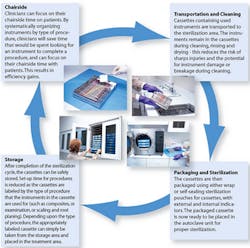
Transport of instruments to the sterilization area
Most dental offices have a designated area for instrument reprocessing that is separate from the dental treatment room. This is ideal, since cleaning, sterilizing and storing instruments in the same room where the delivery of patient care is provided increases the risk of cross-contamination. The removal and disposal of single-use sharps such as needles, blades, orthodontic wires and glass must be done at the point of use, typically in the dental treatment room.
Some instruments and materials are single-use only. Single-use items should be segregated in the operatory, and those that are sharp or otherwise pose a risk of injury must be discarded into a sharps container (Figure 2). Items without risk, such as a saliva ejector, can be thrown into the trash. Finally, the tray or cassette of reusable instruments is taken to the cleaning and sterilization area for processing.
To prevent accidental injury with the contaminated instruments, special handling should be used to transport the instruments to the cleaning and sterilization area.2 The Centers for Disease Control and Prevention (CDC) states that, “Contaminated instruments should be handled carefully to prevent exposure to sharp instruments that can cause percutaneous injury. Instruments should be placed in an appropriate container at the point of use to prevent percutaneous injuries during transport to the instrument processing area.” In addition, the Occupational Safety and Health Administration (OSHA) says, “The person handling the instruments through removal, cleaning, packaging and sterilization needs to use heavy-duty gloves to help prevent injury with sharp contaminated instruments.” Although heavy-duty gloves (utility gloves) may feel more awkward than examination gloves, they provide extra protection while handling instruments during the cleaning, rinsing, drying, packaging and sorting procedures that take place during instrument reprocessing.3 The fine tactile sensitivity needed during dental procedures is not necessary during instrument cleaning and sterilization; therefore, heavy-duty gloves pose no problem in this regard. Additionally, nitrile utility gloves are available in a variety of sizes, allowing a more secure fit.
Cleaning
Using mechanical means of instrument cleaning rather than hand scrubbing should minimize handling of instruments.4 If procedures are used whereby hand scrubbing is necessary, heavy-duty (utility) gloves, mask, eyewear and gown should always be worn while cleaning.5 Minimize the risk of puncture injury by scrubbing only one instrument at a time while holding it low in the sink.
Use of a system utilizing locked cassettes eliminates the need to sort, handle and hand scrub individual instruments - reducing the risk of infection from contaminated instruments - and results in savings of, on average, five minutes during instrument reprocessing, as well as fewer damaged instruments, since the instruments are locked in position during reprocessing (Figure 3). As with any standardized procedure, a standardized instrument reprocessing protocol also results in easy staff training and cross-training.
In general, three classifications of mechanical cleaning devices are available for the dental office. They are the ultrasonic cleaner, instrument washer and instrument washer/disinfector.
Ultrasonic cleaning devices
An ultrasonic cleaner uses sound waves, that are outside the human hearing range to form oscillating bubbles, a process called cavitation. These bubbles act on debris to remove it from the instruments. Some manufacturers also use intermittent or sweeping sound waves to help improve the device’s cleaning ability and to decrease the potential for hot spots in the ultrasonic bath. Specialized detergent formulations are available for the solutions in ultrasonic machines. When selecting a cleaning agent to use in the ultrasonic cleaner, always consider the effect on materials and instruments. Household products are inappropriate because they cause pitting, corrosion, rust or other damage to instruments, and potentially to the ultrasonic chamber. Therefore, it is best to follow the manufacturer’s instructions, thereby choosing a solution that is compatible with the unit and the instruments. The procedure for cleaning the instruments in the ultrasonic cleaner is as follows:
- Suspend instruments in the ultrasonic bath using a rack or basket fitted to the unit.
- Do not lay instruments directly on the bottom of the ultra sonic cleaner, as this can interfere with cleaning and cause damage to instruments and the ultrasonic machine.
- Avoid overloading the ultrasonic device, since that could inhibit its cleaning ability.
It is important to follow the manufacturer’s instructions for the ultrasonic cleaning cycle.
In general, the timer is activated for three to six minutes for loose instruments and ten to twenty minutes for instrument cassettes, and the timing is adjusted as necessary. While the ultrasonic device is running, the lid or cover should be kept on to reduce the release of aerosol and spatter into the area from the ultrasonic cleaner. Routinely replacing the cleaning solution in the ultrasonic machine is important, and is necessary at least once a day, more often with heavy usage.
Instrument washers
Instrument washers use high-velocity hot water and a detergent to clean instruments. Widely used for decades in hospitals and large facilities as part of the central sterilization process, these devices have recently become available for the dental office. These devices require personnel to either place instruments in a basket or to use instrument cassettes during the cleaning and drying cycles. Instrument washers for dental offices come in two different designs. One is a counter-top model. This type does not require professional installation. The other type is built-in and resembles a kitchen dishwasher (Figure 4). It functions much the same as the counter-top model, but it has a larger capacity and requires professional installation. Some models have the ability to dry the instruments after washing, some do not.
Instrument washers/thermal disinfectors
These devices may look like the instrument washers described above; however, there is one important difference. The high temperature of the water and chemical additives in these devices cleans and disinfects the instruments. The significance of this lies in how personnel can handle the instruments after the process. Upon removal from a thermal disinfector, instruments can be more safely handled, and if the dental healthcare professional were to sustain a puncture injury, it would not require the follow-up that a contaminated exposure requires.
All instrument washers and thermal disinfectors use either a detergent or a water-softening agent. It is possible for the pH of some of these chemicals to be incompatible with certain metals in dental instruments. For specific recommendations, the manufacturer of the dental instruments and the manufacturer of the instrument washer should be consulted.
Instrument washers and thermal disinfectors are approved medical devices that have been rigorously tested to meet Food and Drug Administration (FDA) requirements for safety and efficacy of medical devices; household dishwashers are not appropriate for use in a dental office.
Instrument examination and care
Cleaning instruments, provides a good opportunity to examine, replace or remove damaged instruments; lubricate items such as handpieces6; and otherwise prepare instruments for sterilization. Instruments must be dry before packaging - if drying was not part of the cleaning process, time must be taken to dry the instruments completely. High-quality metal dental cassettes specially designed to withstand high temperatures are preferred for use with steam and chemical vapor sterilizers. Most sterilizers on the market today offer a cassette rack, which helps to prevent over-loading in the sterilizer, thereby reducing the risk of ineffective sterilization and ultimately of infection and cross-infection.
Packaging
Packaging used for instruments and cassettes prior to sterilization includes wrap, paper pouches, plastic pouches, combination paper/plastic pouches and nylon tubing. Sterilization packaging is specifically designed to allow penetration of heat, steam or vapor and then to seal the sterilized instruments inside the package for sterile storage (Figure 5). After sterilization, instruments should remain in packages until use. Different materials are appropriate for different types of sterilizers.7, 8 Unless otherwise specified, all packaging is single use only. Using tape to reseal previously used packaging material may inhibit its ability to continue to function as intended by the manufacturer.
Sterilization
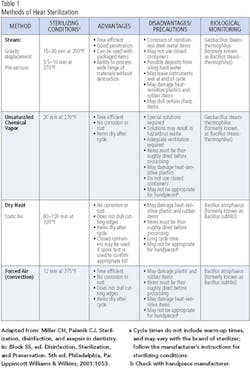
Parameters such as time, pressure and temperature vary according to the type of sterilizer, materials being sterilized and individual models within sterilizer brands. General sterilization parameters for each type of sterilizer appear in Table 1. The first step in determining the settings for the sterilizer is to refer to the manufacturer’s instructions. Sterilizers are medical devices, requiring clearance by the Food and Drug Administration before manufacturers may offer them for sale. The FDA requires rigorous testing to ensure an adequate margin of safety in each cycle type described in the instructions. Failing to follow the instructions of the manufacturer is ill advised, since it may result in inadequate sterilization of the instruments or devices in the sterilizer. It is never appropriate to use a household device, such as a toaster oven, for sterilization of dental instruments, devices, or equipment.
Steam autoclave
Steam autoclaves are the most commonly used type of heat sterilizer in dental practices. Two types of processes employ steam under pressure. The difference between the two is the manner in which the machine evacuates the air from the sterilization chamber and then introduces the steam.
Gravity displacement sterilizers rely on the forces of gravity to force air out of the chamber through air escape vents. The steam entering the chamber from the water reservoir displaces the air as it leaves the chamber. The combination of pressurization of the chamber, steam and a high temperature for a prolonged period has the ability to kill virtually all microorganisms. This is the most common type of autoclave found in dental offices in the United States. A typical cycle for wrapped instruments includes heat-up and pressurization time, followed by a 15-to-30-minute cycle during which sterilization is taking place (121°C at 15 psi). The sterilization cycle time decreases as the temperature is increased. It is important to use cycle times and temperatures described in the owner’s manual, and never to interrupt the sterilization cycle to remove or add items, or for any other reason. Interruption of the cycle will result in instruments that are not sterile and therefore not safe for use on patients. After the sterilization cycle, the sterilizer must depressurize and the packs remain in the sterilizer for drying. The drying phase may take anywhere from 20-45 minutes. The unit must only be opened after completion of the drying cycle. Upon removal from the sterilizer, sterile packs must be stored in a clean, dry area. Packs that become wet, torn, contaminated, or otherwise compromised require resterilization.9
Prevacuum autoclaves (also called Class B or Type B sterilizers) use a variety of technologies to remove air from the chamber before the steam enters, thus creating a vacuum. Most use a pulse vacuum to ensure elimination of air from the chamber. This is generally a more efficient means of pressurizing the chamber; therefore, the operator may notice some minor time saving in the start-up of the prevacuum sterilizers. Most prevacuum sterilizers use a temperature of 132°C-135°C for 3-10 minutes to achieve sterilization. This higher temperature may be unacceptable for some items, such as Teflon-coated instruments. Total time for pressurization, sterilization, venting and drying is generally considerably shorter than that for gravity sterilizers - about 45 minutes.
Dry-heat sterilization (convection and static air)
Dry-heat sterilization employs high temperatures for extended periods to achieve sterilization of instruments. The method of heat circulation in dry-heat sterilizers is usually convection, which helps to ensure that the heat circulates throughout the sterilization chamber during the process. Mechanical convection is more effective; the sterilizer contains a fan or blower that continually circulates the heated air to maintain a uniform temperature throughout the chamber. Most commercially available dry-heat sterilizers on the market today are of this type.
The higher temperature of a dry-heat sterilizer means that paper will scorch and plastic will melt. Specialized packaging material is available for dry-heat sterilizers. Most handpieces will not tolerate the higher temperatures of a dry-heat sterilizer. Mechanically driven handpieces that contain turbines and bearings are susceptible to damage at higher temperatures. The manufacturer’s instructions should be checked for compatibility of instruments, devices, and materials with the unit and the handpiece manufacturer’s instructions should be followed for preparation of the handpiece prior to sterilization and for sterilization itself.
Unsaturated chemical vapor sterilization
Unsaturated chemical vapor sterilization relies upon the use of a proprietary chemical that contains formaldehyde, alcohol and other inert ingredients, instead of water, to produce a vapor to promote the sterilization. Use of this proprietary chemical also results in the vapor having less humidity and therefore being less corrosive to sensitive instruments than if water were used.
Sterility assurance
All the efforts that go into the preparation of instruments are futile if the sterilization process itself is not successful. There is no way of seeing that instruments are sterile by simply observing the sterilizers and packs, even though a chemical or mechanical indicator may have changed. An indicator such as autoclave tape may change color when exposed to heat, but there is a possibility that the heat was not present for the proper length of time or that there was inadequate pressure. Indicators that go on the outside of the packs are useful for identifying processed and unprocessed packs. Failure of sterilization can occur due to mechanical malfunction of the sterilizer or due to operator error. There are several methods to provide assurance of sterility.
Operator error
It is common to rely upon the automated functions of the sterilizer to tell the DHCP if there is a problem with the sterilization process. Most sterilizers have a system to notify the operator of mechanical malfunction, but sterilizers cannot notify the operator whether the contents of the instrument packs or cassettes are sterile or not. Operator error in loading the sterilizer could result in failure to sterilize all the packs in spite of the proper time, temperature and/or pressure. It is important to avoid overloading the sterilizer or loading packs and cassettes on top of one another; use of a cassette system helps to reduce operator error due to overloading. The heat and/or steam must be able to circulate throughout the chamber and between the packs or cassettes for successful sterilization.
Chemical indicators
Chemical indicators indicate the presence of certain conditions during the sterilization cycle, such as the presence of heat and steam.10 There are five classifications of indicators recognized by the FDA, and it is important to note that it is now recommended that all packs or cassettes include internal and external indicators.
Class 1 - Process Indicators. These are placed on the outside of packs and are useful in determining which packs have been properly processed versus those that have not. Class 1 process indicators include autoclave tape and the color change indicators embedded on the outside of sterilization packaging materials.
Class 2 - Bowie-Dick Indicators. These show the pass/fail in prevacuum sterilizers. This test is conducted daily with the chamber empty, during the first cycle of the sterilizer, and is available as a kit from commercial sterilization monitoring companies.
Temperature-Specific Indicators. These react to one of the critical parameters of sterilization and indicate exposure to a specific value such as temperature or psi.
Class 4 - Multi-parameter Indicators. These react to two or more of the critical parameters in the same manner as Class 3 indicators.
Class 5 - Integrating indicators. These are designed to react to all critical parameters of sterilization cycles. When used properly, integrating indicators may serve as the basis for the release of processed items, excluding implants. It is important to follow the manufacturer’s specific instructions for use regarding a test challenge pack.
Biological monitoring
The use of biological monitors (spore tests) is the most reliable method to validate that the sterilizer is functioning and that the sterilization of instruments is effective (Figure 6). These monitors consist of paper strips or vials impregnated with bacterial spores that are specifically resistant to the sterilization process. New spore tests have been developed that enable completion of biological monitoring in-office and yield results in as little as 24 hours. These tests allow quick remediation and validate proper infection control procedures without a long lag time during which the sterilization procedure may have become ineffective but is not known. It is recommended that biological monitoring be conducted at least weekly11 and with every load that includes an implantable device.
Resources
U.S. Department of Labor, Occupational Safety and Health Administration. 29 CFR Part 1910.1030. Occupational exposure to bloodborne pathogens; needlesticks and other sharps injuries; final rule. Federal Register. 2001;66:5317-5325. As amended from and includes 29 CFR Part 1910.1030. Occupational exposure to bloodborne pathogens; final rule. Federal Register. 1991;56:64174-64182. Available at: http://www.osha.gov/SLTC/dentistry/index.html.CDC. Guidelines for Infection Control in Dental Health-Care Settings - 2003.
References
1. www.cdc.gov/mmwr/preview/mmwrhtm/rr5217a1.htm. Accessed August 2006.
2. www.osha.gov/SLTC/dentistry/index.html. Accessed August 2006.
3. Greene VW. Microbiological contamination control in hospitals. 1. Perspectives. Hospitals 1969;43:78-88.
4. CDC. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR 2003;52(No. RR-10). Oper Dent. 2005 Jan-Feb;30(1):16-25.
5. www.osha.gov/SLTC/dentistry/index.html. Accessed August 2006.
6. Monagahn DM et al. The performance of air-turbine handpieces in general dental practice. Compend Contin Educ Dent. 2004;25(1 Suppl):24-29.
7. Association for the Advancement of Medical Instrumentation, American National Standards Institute. Good hospital practice: steam sterilization and sterility assurance. ANSI/AAMI ST46-1993. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1993.
8. Rutala WA, Weber DJ. Choosing a sterilization wrap for surgical packs. Infect Cont Today. 2000;4:64-70.
9. Rosa AC et al. Effects of handling and storage on sterile dental instruments. Acta Odontol Latinoam. 2001;14(1-2):35-39.
10. Association for the Advancement of Medical Instrumentation. Chemical indicators---guidance for the selection, use, and interpretation of results. AAMI Technical Information Report No. 25. Arlington, VA: Association for the Advancement of Medical Instrumentation, 1999.
11. www.cdc.gov/mmwr/preview/mmwrhtm/rr5217a1.htm Accessed August 2006.
Eve Cuny, MS
Eve Cuny is the Director of Environmental Health and Safety and Assistant Professor in the Department of Pathology and Medicine at the University of the Pacific School of Dentistry. She has consulted with the Centers for Disease Control and Prevention, American Dental Association, California OSHA, California Dental Board and other agencies on issues related to safety and infection control in dentistry. She has presented over 100 continuing education programs throughout the world and published numerous articles and textbooks. Ms. Cuny is also founder and managing partner of Eve Cuny Consultants, LLC, a consulting group specializing in product evaluation, professional writing and other services to the dental profession and industry.
Helene Bednarsh, RDH, MPH
Ms. Bednarsh is the Director of HIV Dental for the Boston Public Health Commission and has participated in local, regional, national and international programs on infection control and HIV/AIDS. She is a member of the American Public Health Association and served on the Oral Health Section Council, the American Association of Public Health Dentistry. She served on the Editorial Board of the American Dental Hygienists Association and the Massachusetts Dental Hygienists Association. Ms. Bednarsh was active with the Massachusetts Public Health Association, serving on the planning committee for dentistry and as secretary. She is a member of OSAP and has served on the Board. She was a member of the ADA/DAAC special workgroup on the Management of Occupational Exposure to Bloodborne Pathogens and Evaluation of Occupational Exposure to TB. She is a member of the ADA Wellness Committee/Peers Network for issues related to HIV/AIDS. Ms. Bednarsh co-chairs the Dental Workgroup of the Boston AIDS Consortium.
If you have any questions or comments for the author of this CE course, please send an e-mail to [email protected]. Please reference the course title and author’s name.
Disclaimer
This course has been made possible through an unrestricted educational grant from Hu-Friedy. The authors of this course have no commercial ties with the sponsors or the providers of the unrestricted educational grant for this course.
Reader Feedback
We encourage your comments on this or any ADTS course. For your convenience, an online feedback form is available at www.ineedce.com.