The Point
by Cynthia A. Chillock, RDH, and Tricia Osuna, RDH, BS
Just when you thought it was safe to go back into the office, Congress unanimously passed and signed into law the Needlestick Safety and Prevention Act (PL 106-430) in order to reduce the number of sharps accidents. According to statistics, one in 10 health-care workers in the United States reports needlestick accidents each year. Some studies show approximately 40 percent of the accidents is not reported, so the number is likely much higher.
I can hear the collective groaning from all of you. It's going to take more time and there will be more complaining from the entire staff, especially the doctor. You have to make sense of another standard that requires three PhDs to decipher, and make changes to the systems you currently use — again. In order to avoid making this article your nighttime sleeping pill, I think I'll just tell you a story. Hopefully, it can save you some time and energy setting this up in your office.
Because the revision says we must include "nonmanagerial employees" in the decision process, I added this to the agenda for the next team meeting. I thought it would be really easy to present this information at one of our short, semi-monthly meetings. Wrong! After presenting what I thought was a no-brainer of information, I was not even remotely prepared for the resistance that followed.
Of course, resistance first came from the doctor, who could not believe that the enemy, meaning OSHA, was once again trying to tell him how to run his practice. He snorted with his usual, "This is ridiculous." The assistants were next, wondering why they had to be part of the decision process. After all, management always made the decisions. We had not asked for opinions in the past, and they did not want to spend the time doing research. Besides, if they had to do it, why didn't the front desk person have to do it, too? After all, she went in the back to help with set-up, clean-up and injections when needed.
Next, the other hygienist said there were newer products on the market we needed to investigate that were considered better safety devices.
Boy, did I open a can of worms! Needless to say, we had to do a great deal of homework before we actually came together as a group to discuss our procedures and products currently in use. We tabled the discussion until the next all-afternoon meeting and made a few assignments.
Four weeks later, we all met again for a four-hour team meeting. The front desk person was assigned to take the minutes. She dutifully recorded names, positions, and the tasks assigned in preparation for the meeting. All of the pertinent information was recorded regarding how we had evaluated our processes and products and why we reached the decisions we made. (The recording of the minutes is one of the things required by OSHA.)
The assignments were delegated as follows. The other hygienist, who is very computer-literate, had gone surfing on Web sites to see what information she could get and to find out definitions and what the OSHA update actually said and meant. I investigated the newer products regarding safer needles and syringes. The assistants provided a detailed step-by-step account of how the sharps are handled in the office during any given procedure. The doctor's job was to listen to all the information and help reach a consensus.
Table I.
What we discovered was that — even though we thought we were handling the sharps the same way — we actually had about three different procedures, depending on who was helping. In fact, we discovered the doctor secretly preferred to hand the syringe back to the dental assistant and did not know there was anything wrong with that. The first assistant didn't know she should not be receiving a dirty syringe uncapped from the doctor to recap it herself. The second assistant was placing the rubber needle-recapping device on his side, in order to allow him to do the proper one-handed recapping procedure. The third assistant/front desk person was actually taking the used syringe out of the room and carrying it into the sterilization room before disassembling and preparing the syringe for sterilization.
What a lesson in the lack of communication that was!
Next, we evaluated two interesting safety syringes I had found that seemed to have safer engineering controls. Sultan Chemist, Inc. makes 1SHOT™ Safety Syringe and Septodont, Inc. produces Ultra Safety Plus XL®. We went over the benefits and drawbacks of each syringe, as well as re-evaluate all of our systems.
The process initiated a wonderful brainstorming atmosphere. It gave everyone an equal opportunity to share their concerns and ideas about the products and work procedures. Everyone also had ownership in the final decision, creating a different type of team-building experience.
Now that you've heard my story, here is what the revisions to OSHA's Bloodborne Pathogens Standard actually say. An addition to the "exposure control plan section" requires all employers who have employees with anticipated exposure to blood or other potentially infectious materials to evaluate on an annual basis:
• The entire process of handling sharps from set-up to clean-up
• Safer medical devices that could reduce or eliminate worker exposure
• Record minutes of these meetings, providing details about who was present, job descriptions and how determinations were made
• A "Sharps Injury Log" records needlestick accidents. The log must include the who, what, when, and where of the injury. Now here's the kicker. Since this meeting must include "nonmanagerial employees," the people who actually handle the sharps get to decide which products and procedures will work best for them. What a novel idea! What a great opportunity for the whole team to begin some interaction and get in on the decision-making process. (If the group is too large to include all team members, the practice is required to have a diverse group — with someone representing each area of expertise — included in the decisions.)
The standard states that the employer must adopt safer needle devices to protect against accidental needlesticks, where feasible and commercially available. This does not mean they must buy the new products. It means they must evaluate new products with technological changes. The team must re-evaluate work practice controls that reduce the likelihood of exposure by altering the way a task is performed. They must be educated on recapping as well as safe and effectively engineered products. The standard is careful to state, "There is no one medical device considered appropriate or effective for all circumstances." We now have the opportunity to make some difficult decisions about our own safety and the safety of our patients.
The time when you are most likely to be stuck with a needle is during recapping. It is also the most dangerous time, since the needle has been contaminated with blood and saliva. Unless a specific device is utilized for recapping and all team members are aware of how to use it, the safest method of recapping is the one-handed scoop technique.1 Various devices are available for recapping; however, they too should be tried by all team members during the evaluation process to decide which is ideal for each individual operator. A major safety consideration is that the recapping should be done by the operator of the syringe and not passed on to an auxiliary.
To save time and aggravation, some definitions and the exact wordings of the standard have been added to this article. This information should help you avoid mistakes and get your team on track in complying with the OSHA changes.
One of the best resources is the OSHA Web site at www.osha.gov. It has a section on frequently asked questions, as well as a 22-page printout of the latest bloodborne pathogens regulation with explanations and definitions.
Other good information, which includes an evaluation form from the Training for Development of Innovative Control Technology Project from the University of California at San Francisco, is available at:
www.osap.org/issues/pages/sharps/OSAP-TDICTP.pdf.
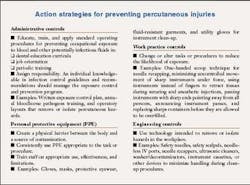
OSAP also offers Sharps Safety: Action Strategies to Prevent Percutaneous Injuries. The OSHA and OSAP printouts could start quite a discussion for your next team meeting. Why not give it a try? It's a great start to getting everyone communicating and involved in the decision-making processes in your office.
Cynthia A. Chillock, RDH, is a clinician, author, lecturer, and consultant. She is president of the Perio-Data™ company and senior consulting editor to Perio Reports Newsletter.She can be reached at (520) 323-1076 or cynthianne@gci-net. com. Tricia Osuna, RDH, BS, is a clinician, lecturer, educator, consultant, and author. She also is the president of Professional Insights, LLC. She can be reached at (310) 370-0849 or [email protected].
References1 Malamed SF: Handbook of Local Anestheia 4th Edition, 97:1032 OSAP Position Paper: Percutaneous Injury Prevention, November 2002Safety syringes with engineering controlsSultan Chemists, Inc.1 SHOT™ Safety Syringe system is the newest of its type on the market today. The beauty of this system is that it is a one-handed technique, which is now recommended by OSHA, CDC, and OSAP. The syringe consists of a sterile, single-use, disposable syringe barrel assembly and a re-usable, autoclavable plunger assembly. The 1SHOT Safety Syringe incorporates a needle retraction and re-advancing mechanism, which may be operated with one hand. It is lighter than traditional syringes and very well balanced. Its engineering allows you a clear view of the cartridge contents due to its clear barrel when aspirating, without changing your technique.
www.sultanchemists.com or (800) 637-8582Septodont, Inc.The Ultra Safety Plus® XL Injection Protection System is a sterile, disposable, single use, reloadable syringe designed to protect the clinician from blood-borne infection. This syringe also contains a bevel indicator and a transparent barrel for improved vision while aspirating and a reusable plunger assembly. This syringe requires a two-handed technique to operate.The plunger is autoclavable for 100 usages.
www.septodontinc.com or (800) 872-8305
• The administration of medication or fluids
• Any other procedures involving the potential for occupational exposure to bloodborne pathogens due to percutaneous injuries from contaminated sharps.Sharps with engineered sharps injury projections (SESIP) mean a non-needle sharp or a needle device used for withdrawing body fluids, accessing vein or artery, or administering medications or other fluids, with a built-in safety feature or mechanism that effectively reduces the risk of an exposure incident. The Sharps Injury Log must contain for each incident:• the type and brand of device involved
• the department or work area where the incident occurred
• an explanation of how the incident occurred.The log is to be kept confidential and on file for employment term plus 30 years, as are all other injury records. It is important to note that if you have less than 11 employees, you are exempt from this record-keeping requirement.
California Dental Associationwww.cdc.gov
Center For Disease Control and Preventionwww.fda.gov
Food and Drug Administrationwww.osha.gov
Occupational Safety and Health Administrationwww.osap.org
Organization for Safety and Asepsis Procedureswww.septodontinc.com
Septodont, Inc.www.sultanchemists.com
Sultan Chemists, Inc.www.tdict.org
Training for Development of Innovative Control Technology Project