Compliance with rotary powered brush
A 15-year review of patient compliance
by Herbert I. Bader, DDS, FACD, FICD
The goals of this retrospective look at 15 years of patient compliance in a periodontal practice are to look at the differences between manual brushing and use of a powered rotary brush as part of daily oral hygiene. Chronic periodontal disease is a serious infection initiated by the presence of multiple pathogens challenging the host immune mechanism.1-5
Unquestionably, the dental hygienist's role in patients' understanding their part in managing an incurable, chronic disease is of paramount concern. As with the patient managing diabetes, the periodontal patient must practice a diligent, daily course of disease control, as well as complying with judicially planned regular recare visits. The inclusion of the patient as part of the management team has been a technique utilized in soft tissue management protocols with considerable success. The goal of these protocols is to initiate the process of reducing the inflammatory burden, prior to proceeding to additional therapy if necessary. The primary caregiver in this scenario is the hygienist, who initiates and co-manages the process.
The role of the plaque biofilm as hosting and supporting the pathogens that initiate the immune response is well established and the subject of considerable literature.6-8 The production of inflammatory mediators initiated by the biofilm pathogens lead eventually to destruction of the supporting tissues in episodes of disease activity or ongoing attachment loss.9-12
What is now known is that these inflammatory mediators disseminate into the blood stream and result in systemic challenges reflected in such diseases as diabetes and chronic CVD, among others.13-20 The systemic spread of such mediators as TNF-alpha, as well as various pro-inflammatory matrix metalloproteinases and other cytokines forms the basis for the "oral/systemic link."21-28 For example, it is well established in the literature that the inflammatory response initiated by periodontal infection is distinctly related to the increased risk for diabetic glycemic events.29-31
The purpose of this retrospective study is to extend the original 10-year observations reported in 2004.32 The compliance history of two groups of cohorts – one using a powered rotary action toothbrush and the other any combination of powered or manual brushes was reviewed.
Patient selection
The subject population of this long-term (15-year) longitudinal study was patients in a private periodontal practice, all of whom had undergone various types of active therapy, and who were initially diagnosed as Type II-III in nature. The therapy ranged from scaling and root planing to surgical intervention – either resective or reconstructive. Both groups received ongoing support and training in daily oral hygiene and, in many instances, received antimicrobial and fluoride-containing agents. Cohorts in the study were selected from a pool of patients who had been in periodontal maintenance over an average of 15.6+/- 1.5 years. Many of these patients had been included in a previous reporting of 10-year observations.
These patients were divided into two groups:
• Group 1: This group consisted of 121 patients, whose mean initial study age was 41.6 +/- 8.7 years, with a male to female ratio of 0.42. All of these patients used an assortment of nonspecified manual and powered brushes, and all received oral hygiene instructions and monitoring at each recare visit.
Figure 1: Rotadent rotary toothbrush used interproximally
• Group 2: This group consisted of 111 cohorts, with a mean study age of 40.3+/-9.6 years, and a male to female ratio of 0.51. All of these patients used a Rotadent rotary powered brush (see Figure 1). All patients received appropriate instruction with the emphasis on linking their daily plaque control with the professional care received at the office.
Patient selection was random with regard to age, sex, and medical and dental history. Medical and/or dental histories were not added as variables in the outcome. Patients were selected by reviewing records in charts accumulated over more than 30 years of periodontal practice. Selections for inclusion were made based on home care appliances being used. All patients were monitored at each recare visit, and received ongoing self-care instructions regardless of which group they were in. Every attempt was made to balance the two groups with regard to the number of teeth present at baseline.
Additional observations included tooth loss in each group throughout the 15-year period. There were no determinations indicated at baseline of periodontal disease severity, or the prognosis for individual teeth, included in the data sets. All clinical observations as to the levels of oral hygiene/plaque control were obtained from the attending hygienist's appointment notes. These were recorded as poor, fair, good, or very good, and were based on the use of two simple clinical indices: The plaque index of Quigley and Hein, and the Loe and Sillness gingival index. In all cases, scores of 0-1 were considered very good or good (acceptable), and all higher scores were either fair or poor, according to the observations.
Another parameter taken into consideration was the temporal compliance with recare visits. For purposes of this report, even though most patients were expected to be seen every three to four months, an interval of six months or less was considered compliant.
The data for this longitudinal report were all compiled according to the groups assigned. The significance of the observed differences in the degree of oral hygiene was interpreted by the clinical judgment of the hygienist. The author retrospectively used standard statistical methods to determine the effect of daily oral hygiene measures on tooth retention. Means and standard deviations of the means summarized quantitative data. The mean tooth loss scores between groups were analyzed using the analysis of variance. A P-value of less than .05 was considered statistically significant.
Results
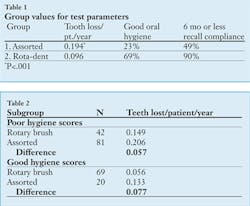
The group values for the test parameters are presented in Table 1. With respect to the rotary tooth-brushing group (0.096 teeth/year/pt) the results interestingly also show that at recall (SPT) 69% of the rotary brush patients and 23% of the assorted group presented with good to very good plaque control/oral hygiene. Table 1 also shows that 49% of the assorted group remained compliant with recare schedules (6 months or less). As opposed to this group, the rotary brush group maintained a 90% level of recare compliance. These clinically observed differences in the level of oral hygiene and recare compliance are clinically significant.
Figure 2: Furcation and with brush head in furcation.
Comparative data for tooth loss in subgroups based on oral hygiene scores can be found in Table 2.
The groups using the rotary brush showed clinically less tooth loss over the 15-year period of observation. Of note, however, is the fact that no statistical difference (P > = 0.05) was recorded in tooth loss between the assorted group of users with good oral hygiene scores ( 0.133 teeth lost/patient/ year) and rotary brush users with poor oral hygiene scores. (0.149 teeth lost/patient/year).
Figure 3: Use in implant maintenance
Generally, the breakdown of the recorded data shows that tooth loss, although overall not extensive, is significantly less for rotary vs. assorted brushers in both compliant and noncompliant subgroups. There is, in fact, no statistically relevant difference in tooth loss between compliant patients in the assorted brushing group and noncompliant patients in the rotary brushing group. What is interesting, as shown by the data, is that the use of the rotary powered brush may compensate for inadequate compliance both in daily disease control and maintenance of recare intervals.
Figure 4: Cleaning in pontic areas
Discussion
The protocols for the therapeutic management of the periodontal diseases include a number of approaches which may include modulation of the host immune system, respectively, and/or regenerative surgical therapies, and anti-infective therapy, both local and systemic.33 The reduction of the pathogenic and inflammatory burdens are not only the most important aspects of the initial phase of active therapy, but are critical in long-term management of the patient.34-36
Whatever approaches are used, and there are many, it is universally agreed that removal of the plaque/biofilm and subsequent detoxification of the root surface is essential. It falls to the role of the dental hygienist as the primary provider of the root detoxification and the education and long-term observation of the patient. The essence of long-term control, however, is daily disease control (plaque control) and supportive periodontal treatment (SPT) on a regular, ongoing basis – again monitored by the hygienist.
The basis by which outcome measurements of successful treatment are measured is generally clinical attachment loss.38-41 A review of the literature shows that most long-term studies of tooth retention in the treated periodontal patient compare the results of surgical with nonsurgical therapy, and there is no real advantage shown by one over the other.42-44
The studies are typically five-year observations, which generally reflect better initial results for pocket depth reduction immediately postsurgically. Over the five-year period of evaluation, the effects of surgical versus nonsurgical intervention tend to coalesce statistically.45 Other observations of long-term tooth loss in periodontal patients enrolled in regular maintenance programs are between 0.06 and 0.96 teeth per year.46-48 A great deal can be learned by using tooth loss as a benchmark for efficacy, and studying the patterns of bone loss.49 The value of inflammatory suppression by nonsurgical means is well demonstrated in a study of more than 1,000 patients over a three-year period.50 The association of root debridement/detoxification and a decrease in tooth mortality is evidenced in this study.
Equally well supported both in the literature and anecdotally by clinicians are the long-term beneficial effects of good periodontal maintenance. The clinical goals of successful maintenance include cessation, or dramatic reduction of bleeding upon probing, and some reduction in pocket probing depth. There are additional benefits to periodontal health that have assumed enormous importance with the emerging understanding of the relationship of oral health to systemic well-being.51-53
Any long-term outcome study of this type must consider, of course, the role of patient compliance. It is, along with regularly scheduled SPT visits, the basic requirement for success. Compliant patients are characterized by their close adherence to daily disease control and their recare schedule.
In this study, 49% of the cohorts using assorted techniques complied with the recommended interval for recare visits. This is opposed to the 90% compliance of patients in the rotary group. The difference between the two groups is significant, not only for the continued dental health of the patients, but the significant impact on patient retention in the recare part of the practice.
This retrospective evaluation of patients in a private periodontal practice looked at the differences between compliant patients (those who regularly kept their SPT appointments while maintaining an acceptable level of daily disease control) using an assortment of nonspecified oral hygiene techniques, and those using a mechanical rotary brush. The data was culled for three different parameters, including tooth loss, level of daily disease control, and compliance with SPT appointments.
As far as tooth loss is concerned, 0.096 teeth/patient/year is in agreement with the literature on tooth retention in the compliant patient in long-term periodontal maintenance. It is clinically relevant as compared with untreated periodontitis patients whose tooth loss may be as much as nine to 10 times higher.54-56 Perhaps the most intriguing results found in the data relate to the level of oral hygiene at SPT visits. The data is both statistically and clinically relevant. The retrospective nature of the study precluded the planned use of specific indices in the protocol.
The observations on these patients were made by the same hygienist over the 15-year period (26-year tenure at the office), demonstrating reasonable consistency without inter-operator variations. Hygienist notes categorized patients into poor, fair, good, or very good based on clinical evaluation at presentation. The rotary brush group of patients exhibited good to very good oral hygiene 69% of the time as opposed to 23% for the assorted group. It is reasonable to posit that this high percentage reflects the patients' feeling of success with their daily disease control. A number of studies have shown that this rotary-powered brush is as effective as a combination of manual brushing, flossing, and interproximal cleaning. It has been shown to be particularly effective interproximally and in furcations where floss cannot be used57-61 (see Figures 2-4).
A most interesting finding relates to the very high percentage of patients in the rotary group who complied with the recommended recare (SPT) intervals, as mentioned earlier. It is important for clinicians to explore those factors that compel a patient to return to the office on a regular basis. Certainly, we could expect reasonably compliant behavior if the fear of failure and/or criticism is removed62 and the need for multiple tasks is reduced.63
It is not unreasonable, therefore, to assume from these results that patients using the rotary-powered brush feel better about their success and are less likely to avoid SPT appointments. The effect of this high level of hygiene retention is extremely valuable to practice growth. The average dental practice has a 50% turnover in patients every five years,64 generally due to patient dissatisfaction. It becomes apparent that patients' feelings of success with their daily regimen is an important part of the equation.
The accepted gold standard for daily disease control has always been brushing and flossing, although few patients floss and brush effectively. The data reported here are not based on such surrogate markers of disease such as the various indices available, but on pragmatic observations by a trained clinician revisiting the same patients over 15 years.
The results suggest that patients who use the rotary-powered brush as dispensed to them in the office as part of a protocol for management, maintain a better level of disease control than patients using an assortment of other techniques. The importance of patient education by the hygienist is emphasized in the results shown here. The data reported in this study may have far-reaching significance for those patients maintaining their periodontal health with a simple, effective instrument. The significance extends to the critically important relationship of oral health to the plethora of systemic entities which could be initiated, prolonged, or exacerbated by ongoing periodontal inflammation.
Herbert I. Bader, DDS, FACD, FICD, is a lecturer in periodontology at Harvard School of Dental Medicine. Dr. Bader is a consultant to Zila, Inc.
References
- Socransky SS, Haffajee AD, et al. Microbial complexes in subgingival mplaque. J Clin Periodontol 1998: 25;134-144.
- Offenbacher S, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 1996 Oct. 67(10 Suppl); 1103-1113.
- Kuula H, et al. Porphyromonas gingivalis-induced periodontitis: local and systemic responses in MMP-8 knock-out mice. Infect Immun 2008 Nov 24 (on line pub ahead of print).
- Listgarten M: Pathogenesis of periodontitis. J Clin Periodontol Dec 2005: 13; 418-425.
- Offenbacher S, et al. Periodontal disease at the biofilm-gingival interface. J Periodontol 2007; 78:1911-1925.
- Haffajee AD, Socransky SS: Introduction to microbial aspects of periodontal biofilm communities, development and treatment. Periodontol 2000 2000;42:7-12.
- Socransky SS, Haffajee AD, et al. Dental biofilms: Difficult therapeutic targets. Periodontol 2000 2002; 28: 12-55.
- Page RC, Offenbacher S, Schroeder HE, et al. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications, and future direction. Periodontol 2000 1997;14:216-248.
- Parkhill J, Branwen J, et al. Association of interleukin-1 gene polymorphisms with early-onset periodontitis. Journal of Clinical Periodontology Volume 27, Issue 9, Date: September 2000, Pages: 682-689.
- Van Dyke TE: Inflammation and periodontal disease: a reappraisal. J Periodontol 2008, 79: 1501-1502.
- Page RC: The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodont Res 1991; 2: 230-242.
- Geismar K, Stoltze K, Sigurd B, et al. Periodontal disease and coronary heart disease. J Periodontol. 2006;77:1547-1554.
- Grau AJ, Buggle F, Heindl S, et al. Recent infection as a risk factor for cerebrovascular ischemia. Stroke 1995;26:373-379.
- Loe H. Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329-334.
- Diabetes and periodontal diseases [position paper]. Committee on Research, Science and Therapy. American Academy of Periodontology. J Periodontol. 2000;71:664-678.
- Liu R, Bal HS, Desta T, et al. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res. 2006;85:510-514.
- Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol.1996;67(suppl):1085-1093.
- Azarpazhooh A, Leake JL: Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77:1465-1482.
- Garcia RI, Nunn ME, Vokonas PS: Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann Periodontol. 2001;6:71-77.
- Dorn JM, Genco RJ, et al. Periodontal disease and recurrent cardiovascular events in survivors of myocardial infarction (MI): The Western New York acute MI study. J Periodontol 2010; 81: 502-511
- Geismar K, Enevold C, et al. Involvement of interleukin-1 genotypes in the association pf coronary heart disease with periodontitis. Of 53 studies, including 4178 cases and 4590 controls. J Clin Periodontol 2008; 35: 754-767
- Nikolopoulos GK, Dimou NL, et al. Cytokine gene polymorphisms in periodontal disease: a meta analysis.
- Struch F, Dau M, et al. Interleukin-1 polymorphism, diabetes and periodontitis: Results from the Study of Health in Pomerania. J Periodontol 2008, 79; 501-507.
- Ferreira SB Jr, Trombone AP, et al. An interleukin-1 beta single nucleotide polymorphism at position 3954 and red complex periodontal pathogens independently, and additively modulate the levels of IL- 1 beta in diseased periodontal tissues. Infect Immun 2008, 76: 3725-373493.
- Geismar K, Enevold C, et al. Involvement of interleukin-1 genotypes in the association of coronary heart disease with periodontitis. J Periodontol 2008, 79: 2322- 2230.
- Taubman MA, Valverde P, et al. Immune response: The key to bone resorption in periodontal disease. J Periodontol 2005; (Suppl) 76: 2033-2041.
- Ford PJ, Gamonal J, et al. Immunological differences and similarities between chronic periodontitis and aggressive periodontitis. Periodontol 2000 2010;53: 111-123.
- Offenbacher S, et al. Rethinking Periodontal Inflammation J Periodontol 2008, Aug 79: 1577-1583.
- King GL: The role of inflammatory cytokines in diabetes and its complications J Periodontol 2008, 79: 1527-1534.
- Covani U, Marconcini S, et al. Relationship Between Human Periodontitis and Type 2 Diabetes at a Genomic Level: A Data-Mining Study. J Periodontol 2009, Vol. 80, No. 8, Pages 1265-1273.
- Campus G, Salem A, et al. Diabetes and Periodontal Disease: A Case-Control Study. J Periodontol 2005, Vol. 76, No. 3, Pages 418-425: 418-425.
- Bader HI: Ten-Year Retrospective Observations of the Impact of a Rotary-Powered Brush vs. Manual Techniques in Periodontal Maintenance. Compendium 2004; 25: 1-7.
- Research, Science and Therapy Committeee of the American Academy of Periodontology: Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. J Periodontol 2001;72: 1790-1800.
- Socransky SS, Haffajee AD: The nature of periodontal diseases. Ann Periodontol 1997; 2: 3-10.
- Van Dyke TE: Inflammation and periodontal diseases: A reappraisal J Periodontol 2008; 79: 1501/1509.
- Kornman KS: Mapping the pathogenesis of periodontitis: A new look. J Periodontol 2008; 79: 1560-1568.
- Garrett JS: Effects of non-surgical periodontal therapy on periodontitis in humans. A review. J Clin Periodontol 1983; 10:515-523.
- Ramfjord SP: Maintenance care and supportive periodontal therapy. Quintessence Int. 1993;24: 465-471.
- Becker W, Berg L, et al. The long term evaluation of periodontal treatment and maintenance in 95 patients. Int J Periodontics and Restorative Dent 1984; 4: 54-71.
- Ramfjord SP, Knowles JW, et al. Longitudinal study of periodontal therapy. J Periodontol 1973; 44:66-77.
- Grossi SG, Zambon JJ, et al. Assessment of risk for periodontal disease. 1. Risk indicators for attachment loss. J Periodontol 1994; 65: 260-267.
- Becker W, Caffesse R, et al. A longitudinal study comparing scaling, osseous surgery, and modified Widman procedures: A result after five years. J Periodontol 2001; 72: 1675-1684.
- Ramfjord SS. Knowles J, et al. Longitudinal study of periodontal therapy. J Periodontol 1973; 44:66-77.
- Isidor F, Karring T, et al. The effect of root planing as compared to that of surgical treatment. J Clin Periodontol 1984; 11: 504-514.
- Antczak-Boukoms A, Joshipura K, et al. Meta-analysis of surgical vs non-surgical methods of treatment from peiodontal disease. J Clin Periodontol 1993;20: 259-268.
- Wilson TG Jr, Glover ME, et al. Tooth loss in maintenance patients in a private periodontal practice. J Periodontol 1987;58: 231-235.
- Matthews TC, Smith CG, et al. Tooth loss in periodontal patients. J Can Dent Assoc 2001; 67: 207-210.
- McFall WT Jr: Tooth loss in 100 treated patients with periodontal disease: A long term study. J Periodontol 1982; 53: 539-549.
- Hirschfeld L, Wasserman B: A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol 1978;49: 225-237.
- Martin JA, Page RC, et al. Tooth loss in 776 treated patients. J Periodontol 2010; 81: 244-250.
- Hujoel PP, Leroux BG, et al. Non-surgical periodontal therapy and tooth loss. J Periodontol 2000; 71:735-742.
- Dissick A, Redman RS, et al. Association of periodontitis with rheumatoid arthritis: A pilot study. J Periodontol 2010;81: 223-230.
- Friedwald VE, Kornman KS, et al. The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: Periodontitis and Atherosclerotic Cardiovascular Disease. J Periodontol 2009;89: 1021-1032.
- Genco RJ, Grossi SG, et al. A Proposed Model Linking Inflammation to Obesity, Diabetes, and Periodontal Infections. J Periodontol 2005;76: 2075-2084.
- Buckley LA, Crowley MJ, et al. A longitudinal study of untreated periodontal disease. J Clin Periodontol 2002; 11: 523-530.
- Konig J, Plagmann HC, et al. Tooth loss and pocket probing depths in compliant periodontally treated patients. J Clin Periodontol 2002; 29: 1092-1100.
- Becker W, Berg L, et al. Untreated periodontal disease: A longitudinal study. J Periodontol 1979; 50: 234-244.
- Bader HI: Comparative efficacy of two powered brushes in interproximal areas. J Dent Res 2008 Abstract #3448.
- McLey L, Sarker S, et al. Model to assess interproximal cleaning by four powered brushing instruments (Abstract) J Dent Res 1995; 74.
- Bader HI, Boyd RL: Comparative efficacy of a rotary and a sonic powered toothbrush on improving gingival health in treated adult periodontitis patients. Am J dent 1999; 12: 143-147.
- Boyd RL, Murray P, et al. Effect on periodontal status of rotary electric toothbrushes vs. manual toothbrushes during periodontal maintenance.1.Clinical results J Periodontol 1989; 60: 390-395.
- Bader HI, Williams RC: Comparative efficacy of two electric brushes in furcations and interproximal areas. J dent Res 1993; Abstract #2166.
- Melamed BG, Bennett CG, et al. Dentist's behavior management as it affects compliance and fear in pediatric patients. J Am Dent Assoc 1983; 106: 324-330.
- DeMatteo MR, DiNicola DD: Achieving patient compliance: The psychology of the medical practitioner's role. New York, NY: Pergmon Press; 1982.
- Golden LM: Denial of dental problems by patients. Ann Dent 1964; 23: 103-110.
Past RDH Issues