Antineoplastic agents and their oral adverse effects
Editor's note: This is part two of a two-part series. Read part one here.
While antineoplastic agents are designed to be toxic to cancer cells, they may also be toxic to other “normal,” rapidly dividing cells. Since these agents interfere with the growth of cells in the lining of the mouth, they slow the ability of oral tissue to repair itself, making the mouth a prime target for the adverse effects of antineoplastic agent therapy.
In this two-part column, we cover the oral adverse effects of antineoplastic agent therapy. In the first installment, we focused on one of the most devastating complications to arise from such therapy—oral mucositis. In the second installment, we will explore other oral adverse effects of antineoplastic agent therapy that may also seriously compromise a patient’s health and quality of life: opportunistic infections, hyposalivation, taste disturbances, bleeding, and neurotoxicity.
Opportunistic infections
Antineoplastic therapy negatively affects bone marrow, resulting in neutropenia and, thus, the development of opportunistic infection. These infections may be commonly caused by bacteria, fungi, and viruses.1 While the typical symptoms of bacterial infection, erythema, edema, and purulence may be present, it is also common for a previously asymptomatic tooth to suddenly give rise to symptoms of infection.2 Gram-positive organisms are often a source of infection in neutropenic patients. Streptococcus viridans may cause bacteremia, while Staphylococcus aureus may cause parotid sialadenitis. Systemic antibacterials, such as amoxicillin or clindamycin, are often used to treat such bacterial infections.3
Also by the author: Local anesthetics, analgesics, and antibacterial agents in pediatric dentistry
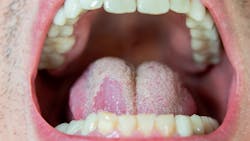
Unfortunately, the use of broad-spectrum antibacterials may, in turn, create a favorable environment for fungal infection. Candida albicans is often detected in these infections.4 Erythematous, pseudomembranous, and hyperplastic candidiasis are typically present and may cause dysgeusia and xerostomia, a burning sensation and general oral discomfort. Topical oral antifungal agents, such as nystatin, are often employed but may have limited efficacy. Systemic agents, such as fluconazole, should be used for persistent fungal infections (especially if the patient is immunocompromised).
In addition to bacterial and fungal infections, patients receiving antineoplastic therapy are at risk for viral infections, including herpes simplex and varicella zoster. The extent of the infection depends on the degree of the patient’s immunosuppression. Since these infections are often the result of reactivation of an existing virus, prophylaxis with antiviral medications may reduce the incidence of infection.5 The first choice of treatment is acyclovir orally.6
In addition, postherpetic neuralgia may be present in 6%–18% of patients over 60 years old.7 Treatment includes topical anesthetics, such as lidocaine. However, if topical anesthetics are not sufficient for pain relief, systemic analgesics are usually employed.8 Opioid analgesics, gabapentin, and antidepressants are typically used in patients receiving antineoplastic therapy, since nonsteroidal anti-inflammatory drugs (NSAIDs) may cause bleeding and directly damage gastric mucosa.
Hyposalivation
Xerostomia may occur in patients receiving antineoplastic therapy due to suppression of salivary function, but it is almost always temporary, so treatment is usually palliative. Xerostomia results in increased salivary viscosity, impaired lubrication, and moistening of the oral mucosa, making it prone to trauma and oral mucositis.5
In addition, xerostomia results in difficulty maintaining oral hygiene due to the loss of salivary antimicrobial proteins and mineralizing components. This increases the risk for dental caries and erosion. Xerostomia should be managed with saliva substitutes. Caries resistance can be enhanced with the use of topical fluorides and remineralizing agents, which are high in calcium phosphate and fluoride.
Taste disturbances
While not seen as severe, xerostomia and other oral complications, such as dysgeusia and dysphagia, may be equally devastating for patients on antineoplastic therapy. These complications affect eating and communication, the most basic of human activities, which can have a significant impact on a patient’s quality of life. Dysgeusia can be quite problematic for patients who are receiving antineoplastic therapy.9 Patients may experience unpleasant taste due to direct toxicity to the cranial nerves, oral mucosa, and taste buds as well as xerostomia and infection. This symptom is generally reversible in patients receiving antineoplastic therapy, and taste sensation returns to normal in the ensuing months. Dysphagia is also common in cancer patients and can predispose them to aspiration and potentially life-threatening pulmonary complications.10
Dysgeusia and dysphagia may lead to unfavorable dietary changes and decreased oral intake, which may result in dehydration, malnutrition, delayed wound healing, and further decreased resistance to infection. Xerostomia and hyposalivation also contribute to these issues. Difficulties with speaking, eating, and drinking socially isolate patients and significantly decrease their quality of life. Patients can become withdrawn and depressed because of the difficulties they encounter living with these oral complications.
Bleeding
In addition to neutropenia, bone marrow suppression may also be responsible for excessive bleeding. Patients may present with petechiae, hematomas, or ecchymoses. Although rarely serious, oral bleeds may also be a concern for patients who are receiving antineoplastic therapy. Areas such as the soft palate, floor of the mouth, lower lip, and vestibular mucosa are vulnerable to hemorrhage.11 Unfortunately, it is still sometimes common for these patients to be told to avoid the use of toothbrushes and dental floss when their platelet counts are low. But the lack of routine oral hygiene may increase the risk of plaque accumulation and, consequently, the risk of local and systemic infection.
Neurotoxicity
Certain antineoplastic agents can cause neurotoxicity. This may result in deep, throbbing oral pain that, unfortunately, is also consistent with pain resulting from acute dental pathology. If neurotoxicity is the diagnosis, it typically resolves within a week of discontinuing the antineoplastic therapy. Pain causes increased morbidity, reduced performance status, increased anxiety and depression, and diminished quality of life. Management of oral pain is particularly challenging because eating, speaking, swallowing, and other motor functions of the head and neck and oropharynx are constant pain triggers. Pain management usually involves the use of opioid analgesics.1
Since many cancer patients are unaware that antineoplastic agent therapy may affect their oral tissues, dental hygienists are invaluable in managing and preventing the oral complications of these agents. These complications may seriously compromise a patient’s health and quality of life and lead them to avoid seeking routine dental hygiene treatment at a time when they need it the most.
Editor's note: This article appeared in the May 2022 print edition of RDH magazine and has been updated as of February 2025. Dental hygienists in North America are eligible for a complimentary print subscription. Sign up here.
References
- Poulopoulos A, Papadopoulos P, Andreadis D. Chemotherapy: oral side effects and dental interventions—a review of the literature. Stomatological Dis Sci. 2017;1:35-49. doi: 10.20517/2573-0002.2017.03
- van Dalen EC, Mank A, Leclercq E, et al. Low bacterial diet versus control diet to prevent infection in cancer patients treated with chemotherapy causing episodes of neutropenia. Cochrane Database Syst Rev. 2016;4(4):CD006247. doi:10.1002/14651858.CD006247.pub3
- Lark RL, McNeil SA, VanderHyde K, Noorani Z, Uberti J, Chenoweth C. Risk factors for anaerobic bloodstream infections in bone marrow transplant recipients. Clin Infect Dis. 2001;33(3):338-343. doi:1086/322595
- Maheronnaghsh M, Tolouei S, Dehghan P, Chadeganipour M, Yazdi M. Identification of Candida species in patients with oral lesion undergoing chemotherapy along with minimum inhibitory concentration to fluconazole. Adv Biomed Res. 2016;5:132. doi:4103/2277-9175.187394
- López BC, Esteve CG, Pérez MGS. Dental treatment considerations in the chemotherapy patient. J Clin Exp Dent. 2011;3(1):e31-e42. doi:10.4317/jced.3.e31
- Lerman MA, Laudenbach J, Marty FM, Baden LR, Treister NS. Management of oral infections in cancer patients. Dent Clin North Am. 2008;52(1):129-153. doi:1016/j.cden.2007.10.006
- Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:1056/NEJMoa051016
- Dubinsky RM, Kabbani H, El-Chami Z, Boutwell C, Ali H, Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63(6):959-965. doi:1212/01.wnl.0000140708.62856.72
- Imai H, Soeda H, Komine K, Otsuka K, Shibata H. Preliminary estimation of the prevalence of chemotherapy-induced dysgeusia in Japanese patients with cancer. BMC Palliat Care. 2013;12(1):38. doi:1186/1472-684X-12-38
- Schindler A, Denaro N, Russi EG, et al. Dysphagia in head and neck cancer patients treated with radiotherapy and systemic therapies: literature review and consensus. Crit Rev Oncol Hematol. 2015;96(2):372-384. doi:1016/j.critrevonc.2015.06.005
- Rahnama M, Madej-Czerwonka B, Jastrzebska-Jamrogiewicz I, Jamrogiewicz R. Analysis of the influence of parenteral cancer chemotherapy on the health condition of oral mucosa. Contemp Oncol (Pozn). 2015;19(1):77-82. doi:5114/wo.2014.45291
About the Author

Tom Viola, RPh, CCP
With more than 30 years’ experience as a board-certified pharmacist, clinical educator, professional speaker, and published author, Tom Viola, RPh, CCP, has earned the reputation as the go-to specialist for making pharmacology practical and useful for dental teams. He is the founder of Pharmacology Declassified and is a member of the faculty of more than 10 dental professional degree programs. Viola has contributed to several professional journals and pharmacology textbooks, and currently serves as a consultant to the American Dental Association’s Council on Scientific Affairs.