Throughout my lengthy career, I thought I had seen just about everything. Then, along came the pandemic. Thankfully, those of us in dentistry have been following Centers for Disease Control and Prevention (CDC) PPE recommendations for decades. I don’t know about the rest of you, but I was anxious upon returning after our seven-week lockdown. So, into solution-based mode I went.
I knew I had PPE covered, but what if I could do more? So, I did: Preprocedural rinsing before beginning procedures. Here is data supporting the use and efficacy of ioRinse, a molecular iodine product I have known about for several years but is now more important than ever.
A number of rinses marketed to dental offices claim (or imply) that they are effective in reducing or eliminating SARS-CoV-2 in the oral cavity. Indeed, the American Dental Association (ADA) initially recommended 1.5% hydrogen peroxide and 0.2% povidone iodine for use as an antiviral prerinse.
When actual antiviral testing of these rinses on SARS-CoV-2 finally did become available, a different picture began to emerge. This new evidence challenges our widely held beliefs on the actual antiviral efficacy of several of these rinses and the conditions under which the testing has been performed.
Consider the following: at the same time that the ADA was recommending 1.5% hydrogen peroxide as an antiviral prerinse, the CDC was advising that 1.5% hydrogen peroxide requires 18 to 20 minutes to inactivate rhinovirus, the virus that causes the common cold.1
As a result of this research, and additional in-vitro and in-vivo testing, Public Health Ontario, the Royal College of Dental Surgeons, and the Canadian Dental Hygienists Association have all advised their constituents to discontinue use of hydrogen peroxide as an antiviral prerinse.3
What are we to believe? And do antiviral oral rinses really perform as well in the presence of saliva as they do in the laboratory? Let’s examine the evidence.
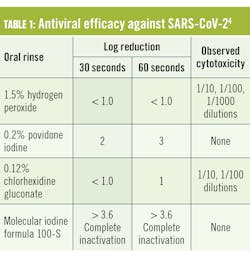
In August 2020, the Antiviral Research Institute of Utah State University conducted a study of the antiviral efficacy of several oral rinses against SARS-CoV-2 (table 1).4
Of the tested rinses, 1.5% hydrogen peroxide and 0.12% chlorhexidine gluconate were poorly effective, even after 60 seconds’ exposure. While 0.2% povidone iodine fared somewhat better, the only rinse to completely inactivateThat testing was not done in the presence of saliva. Additional testing performed at several independent laboratories yielded the following results5 (table 2).
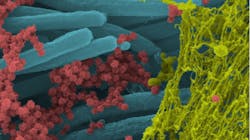
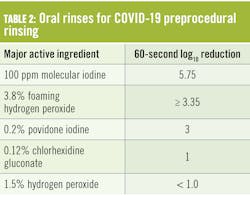
The 3.8% hydrogen peroxide oral rinse was tested on coronavirus strain #229E, not SARS-CoV-2. The other rinses were all tested on SARS-CoV-2. All testing occurred at 60 seconds’ exposure. As noted in the table, the rinses varied in efficacy from poorly effective to highly effective, with the molecular iodine rinse being the most effective. These tests did not include saliva as an organic load. Saliva and mucous from the respiratory tract can cover, coat, and protect viruses from exposure to antiseptic rinses. Saliva can also directly neutralize antiseptics (figure 1).So, how did molecular iodine, the best performing rinse in the absence of saliva, fare in the presence of saliva? It was tested on fresh human saliva at a volume 50% greater than the resting saliva volume (i.e., the volume of intraoral saliva just before swallowing) found in the average adult. Where the molecular iodine rinse had achieved a 5.75 log kill in the absence of saliva, at 60 seconds, its virucidal efficacy at 60 seconds in the presence of saliva was reduced 8.7% to a 5.25 log kill.
This biocidal efficacy in the presence of saliva is remarkably high (far higher than any of the other tested rinses, without a salivary organic load). It is particularly impressive considering that a number of other oral rinses are neutralized in the presence of saliva.
Right now, a molecular iodine rinse is the clear winner as a prerinse for SARS-CoV-2. It would be helpful for other rinse manufacturers to have their products tested in the presence of saliva, so that, if also shown to be effective, dental offices could choose among several rinses that were tested under realistic conditions. As a conscientious health-care provider, this is my product of choice moving forward.
References
- Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf
- Ortega KL, Rech BO, El Haje GLC, Gallo CB, Pérez-Sayáns M, Braz-Silva PH. Do hydrogen peroxide mouthwashes have a virucidal effect? A systematic review. J Hosp Infect. 2020;106(4):657-662. doi: 10.1016/j.jhin.2020.10.003
- Don’t use hydrogen peroxide as a COVID-19 pre-procedural rinse, experts say. Dental Tribune Canada. December 12, 2020. https://ca.dental-tribune.com/news/dont-use-hydrogen-peroxide-as-a-covid-19-pre-procedural-rinse-experts-say/
- Moskowitz H, Mendenhall M. Comparative analysis of antiviral efficacy of four different mouthwashes against severe acute respiratory syndrome coronavirus 2: an in-vitro study. Int J Experiment Dent Sci. 2020;9(1):1-3. doi: 10.5005/jp-journals-10029-1209
- Clinicians Report. April 2021. Volume 14, Issue 3, Pages 1-3.
- Ehre C. SARS-CoV-2 Infection of Airway Cells. N Engl J Med. 2020;383(10):969. doi:10.1056/NEJMicm2023328
Eva Perry, RDH, is a 38-year veteran hygienist who has experienced many changes throughout her time practicing. She has had many wonderful teachers, employers, and mentors in diverse areas of dentistry ranging from general practice, pediatric, prosthodontic, orthodontic, and periodontic specialties. Studying the profound impact of molecular iodine technology in each of these dental specialties is an exciting part of her professional future.
About the Author

Eva Perry, RDH
Eva Perry, RDH, is a 38-year veteran hygienist who has experienced many changes throughout her time practicing. She has had many wonderful teachers, employers, and mentors in diverse areas of dentistry ranging from general practice, pediatric, prosthodontic, orthodontic, and periodontic specialties. Studying the profound impact of molecular iodine technology in each of these dental specialties is an exciting part of her professional future.