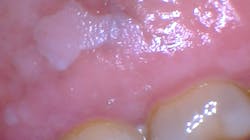
Do you have a patchy understanding of leukoplakia?
Leukoplakia is a somewhat mysterious condition in the field of dentistry. In this article, we’ll examine factors linked to leukoplakia, how the condition typically presents, and why dental professionals should care.
Defining leukoplakia
Clinically speaking, leukoplakia is a term for a white patch: leuko meaning “white,” and plakia meaning “patch.” Although any oral lesion is concerning, leukoplakia is also classified as a potentially premalignant disorder. In other words, leukoplakia denotes a state of existence that must be determined by the exclusion of causes. The extreme worry begins when all possible causes have been ruled out and we’re left with leukoplakia but no known etiology. What’s the next course of action, and what protocol is acceptable? Leukoplakia must be treated as a precancerous entity and is classified by the World Health Organization (WHO) as potentially premalignant.1
Leukoplakia specimens are often described by clinicians as being asymptomatic, white, red, or speckled (mixed), and they can have varying surface textures such as smooth, rough, pebbled, a thick keratin layer (hyperkeratosis), corrugated, or they may be fissured and may feature any of these combinations. These lesions are first diagnosed clinically through visual examination and palpation. Leukoplakia usually occurs in individuals over 40 years old, in less than 1% of the population with a 2%–3% annual risk of transformation. The worldwide prevalence is estimated at 1.5%–4.3%, but may be as high as 60% among the tobacco-smoking population.2,3
Approximately 9% to 34% of all leukoplakias present with dysplasia or squamous cell carcinoma.4,5 These lesions are typically found on the lip, tongue, vermilion border, gingiva, and buccal mucosa. There is a strong male predilection, but depending upon tobacco use, this may vary, as some females use as much or more tobacco products in various countries.3 The only profession that has a noted increase of leukoplakia is glassblowers.4 With these facts in mind, leukoplakia becomes a disease diagnosed by the exclusion of any other known entities. But, because of the altered tissue and the fact that leukoplakia has a higher rate of malignant transformation, it is considered precancerous. Determining the etiology and establishing a protocol for repair is necessary during clinical evaluation.
There have been studies documenting the usual etiology or suspects in what has been designated leukoplakia with tissue specimens submitted for biopsy confirmation. The lesions may also be histologically classified as homogeneous or nonhomogeneous.3 Some lesions are described as uniformly white with a thick surface that is flat or slightly wrinkled and homogeneous. Nonhomogeneous lesions may be white, red, ulcerated, or with a pebbly or verrucous surface. Additional categories used for these nonhomogeneous characteristics are: speckled leukoplakia (erythroleukoplakia), verrucous leukoplakia, and proliferative verrucous leukoplakia that has a high rate of progression to squamous cell carcinoma. The biopsy report of specimens submitted with a leukoplakia clinical diagnosis may be termed hyperkeratosis in approximately 80% of specimens as a pathology diagnosis. The remaining 20% will show some grade of dysplasia, cancer in situ, or invasive squamous cell carcinoma.3 These cellular changes are abnormal cells within tissue that are only identified through biopsy and microscopic evaluation. Precancerous-type lesions must be monitored when the initial cause cannot be determined.4 The suspected area may ultimately become malignant.
Factors linked to leukoplakia
In the effort to exclude possible etiology for a white patch, there are some known suspects that are most often considered in searching for the etiology of white tissue changes.
- Frequent alcohol use
- Ultraviolent radiation
- Proliferative verrucous leukoplakia3 has a high probability to progress to squamous cell carcinoma. These are nonhomogeneous lesions.
- Presence of microorganisms such as Candida albicans and human papillomavirus (HPV)
- Mechanical injury from chronic chewing on edentulous surfaces, especially the retromolar trigone. Broken teeth and faulty restorations may contribute to chronic irritation as well.5
- Cinnamon-induced stomatitis. Some documentation has been published linking chronic use of cinnamon products and flavoring agents to leukoplakia.6,7 These are commonly found in mints, chewing gum, and many foods and drinks.
- Use of products containing the herbal extract sanguinaria (associated with keratoses)8
- Tobacco use, including all forms of the product, smoked or chewed. Additionally, betel quid, toombak, snuff dip, and those patients using reverse-type smoking techniques which are very popular with certain ethnic groups/countries.9–11 Chronic use of tobacco products results in a thick, fissured, leathery white plaque.
- Lichen planus—the reticular pattern and the plaque form present as white lesions
- Lichenoid reactions resulting from some medications and dental restoration reactions
- White sponge nevus
- Benign migratory glossitis, which may present as both yellow and white areas
- Leukoedema, presenting with a varied blue/white appearance. In some cases, the leukoedema may appear thicker and whiter.
- Chemical burns such as aspirin and even garlic placed on the tissue (some cultures may use garlic as a healing agent for oral lesions). Over- the-counter medications containing eugenol or other chemicals.
- Presence of Candida albicans and HPV infection
- Erythroleukoplakia or speckled leukoplakia (often advanced dysplasia)
- Morsicatio buccarum (chronic injury/cheek chewing) and frictional keratosis; constant tissue injury and inflammation may produce cell changes long term. May involve tongue- and lip-chewing (morsicatio labiorum) as well.
- Psoriasis, which may present in various red or white tissue changes
Considerations
Some suggest having the patient see a specialist, such as those in oral surgery, oral pathology, or oral medicine, and a team of specialists including an otolaryngologist, dentist, primary provider, nurse practitioner, and a pathologist when a premalignant diagnosis is made. Carrard makes the point that most general dentists are not trained specifically in taking biopsies and in what is called “mapping” or multiple biopsies from different sites in extensive leukoplakia.4 The accuracy of a biopsy depends on the diagnostic skills of the clinician. Patients may need to be seen on a regular basis and also monitored for tissue changes long term when a white patch is noted or a biopsy indicates a premalignant or malignant state.
In conclusion
Leukoplakia is often mentioned as a clinical diagnosis, but as discussed previously, the term is only a description meaning “white patch,” and the etiology of other factors must be excluded. Leukoplakia is a clinical diagnosis, and the histologic spectrum can go from hyperkeratosis to frank carcinoma. This clinical diagnosis means it is potentially premalignant and must be viewed that way. At the time of biopsy, even nondysplastic leukoplakias undergo malignant transformation, albeit at a markedly lower rate, than those with obvious dysplastic change.
Dental professionals should care about any oral lesion because without the exclusion of other possible factors, the patient may develop a future malignancy that could have been discovered and possibly prevented much earlier, if treated successfully. Excluding factors does take time and is not always easy. Follow-up on any presence of leukoplakia is needed, as with any tissue that has changed or has the possibility of being altered in the future. Often, a specific etiology is not present, and the term “leukoplakia” is the most fitting classification. Since leukoplakia is considered a premalignant state, referring a patient to a specialist may be the best solution when there is no known etiology. Frequent biopsy or mapping can be performed.
As always, ask smart questions and continue to listen to your patients.
Editor's note: This article appeared in the August 2021 print edition of RDH magazine.
REFERENCES
- W Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575-580. doi:10.1111/j.1600-0714.2007.00582.x
- Neville BW, Damm DD, Allen CM, Chi AC. Oral and Maxillofacial Pathology. 4th ed. Elsevier; 2016:355-364.
- Wright JM. Chapter 13: White lesions. In: General and Oral Pathology for the Dental Hygienist. 3rd ed. Jones & Bartlett Learning; 2019:343-347.
- Carrard VC, van der Waal I. A clinical diagnosis of oral leukoplakia; A guide for dentists. Med Oral Patol Oral Cir Bucal. 2018;23(1):e59-e64. doi:10.4317/medoral.22292
- Natarajan E, Woo SB. Benign alveolar ridge keratosis (oral lichen simplex chronicus): A distinct clinicopathologic entity. J Am Acad Dermatol. 2008;58(1):151-157. doi:10.1016/j.jaad.2007.07.011
- Endo H, Rees TD. Cinnamon products as a possible etiologic factor in orofacial granulomatosis. Med Oral Patol Oral Cir Bucal. 2007;12(6):E440-E444.
- Allen CM, Blozis GG. Oral mucosal reactions to cinnamon-flavored chewing gum. J Am Dent Assoc. 1988;116(6):664-667. doi:10.14219/jada.archive.1988.0003
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(4):455-464. doi:10.1016/s1079-2104(00)70125-9
- Ahmed HG. Etiology of oral cancer in the Sudan. J Oral Maxillofac Res. 2013;4(2):e3. http://doi.org/10.5037/jomr.2013.4203
- Mustafa MB, Hassan MO, Alhussein A, Mamoun E, El Sheikh M, Suleiman AM. Oral leukoplakia in the Sudan: clinicopathological features and risk factors. Int Dent J. 2019;69(6):428-435. doi:10.1111/idj.12509
- Babiker TM, Osman KA, Mohamed SA, Mohamed MA, Almahdi HM. Oral cancer awareness among dental patients in Omdurman, Sudan: a cross-sectional study. BMC Oral Health. 2017;17(1):69. doi:10.1186/s12903-017-0351-z
About the Author
Nancy W. Burkhart, EdD, MEd, BSDH, AAFAAOM
NANCY W. BURKHART, EdD, MEd, BSDH, AAFAAOM, is an adjunct professor in the Department of Periodontics-Stomatology, College of Dentistry, Texas A&M University, Dallas, Texas. She is founder and cohost of the International Oral Lichen Planus Support Group (dentistry.tamhsc.edu/olp/) and coauthor of General and Oral Pathology for the Dental Hygienist, now in its third edition. Dr. Burkhart is an academic affiliate fellow with the American Academy of Oral Medicine, where she also serves as chair of the Affiliate Fellowship Program Committee. She was awarded the Dental Professional of the Year in 2017 through the International Pemphigus and Pemphigoid Foundation, and she is a 2017 Sunstar/RDH Award of Distinction recipient. Her professional interests are in the areas of oral medicine and the relationship between oral and whole-body health, with a focus on mucosal disease and early oral cancer detection. Contact her at [email protected].