A practical guide for testing dental unit waterlines: Part 1
In many areas of dentistry, outcomes are determined by the dental health-care professional’s (DHCP) level of ethics, standard of care, and ability to stay up to date. Dentistry is not static; being aware of current findings and embracing them ethically can protect both the patient and DHCP from disease. Dental unit waterlines are one of these areas. When new findings and regulations come forward, finding out if you are in compliance can be daunting—like opening a can of worms. Finding out you have an issue and addressing it can be a defining moment.
What do we know?
Recently, there has been a focus on water safety in dentistry. Dental unit waterline systems (DUWS) have long been known as a harbor for high concentrations of potentially pathogenic bacteria.1 The small tubing has proven a haven for specific pathogenic bacteria,2 including Pseudomonas spp., Legionella spp., and nontuberculosis mycobacteria.3 Most microorganisms detected in DUWS come from public water supply and do not pose a risk for healthy patients. Until recently, it was assumed infection was possible only in immunocompromised patients. But infection outbreaks involving at least 90 healthy patients in Anaheim, California, and Atlanta, Georgia, suggest otherwise. There are now documented cases of direct transmission of disease from DUWS.4–10
Recent cases of disease acquisition from dental waterlines involving healthy children have also brought the topic to the forefront in dentistry and the media.11,12 DHCPs may also be put at risk by exposure to water spray and aerosols from devices that use DUWS.
Related reading
Dental unit waterlines: Trickier than you think
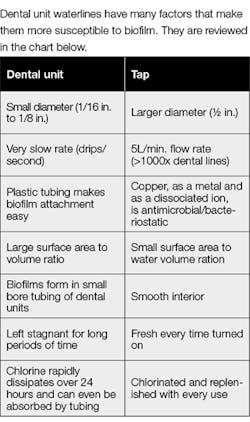
DUWS can harbor biofilm, a reservoir for pathogens. The smaller the tubing, the greater the chance of biofilm formation. Many studies have shown that evidence-based practical methods for controlling microbial contamination are urgently needed. With the desired level of colony-forming units (CFU) being the same or less than drinking water,13 DHCPs need to verify they are using water that meets or exceeds Environmental Protection Agency (EPA) standards for drinking water (i.e., less than or equal to 500 CFU/mL of heterotrophic water bacteria) for nonsurgical treatment output water. Some manufacturers require as low as 200 CFU/mL for specific units. Know the details of your individual unit.
The impact of biofilm
Microorganisms such as bacteria have the ability to survive by creating a sticky adhesive material called biofilm and attaching to surfaces. Biofilm allows the bacteria to adapt to their environment, especially a hostile one (e.g., running water).14
Microorganisms in biofilm “have been shown to elicit specific mechanisms for initial attachment to a surface, development of a community structure and ecosystem, and detachment,” according to a 2002 article in Emerging Infectious Diseases.15 “Biofilm provides an optimal environment for the exchange of genetic material between cells” and communication using other chemical signals.15
Under the protection of biofilm, microbes become tolerant and resistant to antibiotics, disinfectants, and human immune responses, increasing difficulty of clinical treatment. The interface between a solid surface (e.g., a pipe or waterline) and a liquid provides the perfect environment for the attachment and growth of microorganisms.15 Biofilm in the water system is highly complex and not removed by gentle rinsing, and is thus considered “irreversibly associated” with a surface.15
Biofilm accumulation has been shown to be concern with DUWS. Small-bore flexible plastic tubing in dental devices can be coated with well-established biofilms. Most of the flow occurs in the center of the tubing, leaving a thin layer of liquid virtually undisturbed at the edges. Add to that periods of recurrent stagnation (e.g., weekends and holidays) and you have the perfect combination for microflora to establish tenacious adherent communities. As the ever-active biofilm thrives, it becomes a source of microbial contamination of DUWS. These microbes detach and can enter the human body via an oral wound or aerosolization. Keeping the patient and DHCP safe requires water that meets or exceeds the drinking water standard. Understanding the nature of biofilm, and most importantly, how to effectively evaluate and eliminate it, is key to reducing risks.2
Oversight and resources
Knowing the recommendations and standards set forth by agencies regulating dental waterline safety can help you and your office understand exactly how to be in compliance and safeguard yourselves from disaster.
FDA: The Food and Drug Administration (FDA) regulates the sterilization of reprocessed medical devices. The dental unit is considered a medical device. DHCP have to read the instructions for use (IFU) provided by the manufacturer of the specific device. In 2015 the FDA stated, “Manufacturers of reusable medical devices are responsible for having labeling that bears adequate directions for use, including instructions on preparing a device for use.” The FDA then recognized the critical role and responsibility of the device-user community to follow the validated reprocessing instructions.17 Every FDA-approved device comes with IFU. It is important to know the directions that keep the patient and DHCP safe, teach those directions, and follow those directions each and every time. It is not a choice.
CDC: The Centers for Disease Control and Prevention (CDC) recommends treating the water used in dental units with commercial products, such as chemical germicides, to meet drinking water standards of less than or equal to 500 CFU/mL.17
ADA: The American Dental Association (ADA) recommends routine monitoring of the water to demonstrate levels of bacteria less than 500 CFU/mL. They also recommend following IFU and monitoring water quality.18
OSAP: The Organization for Safety, Asepsis and Prevention (OSAP) focuses on strategies to improve compliance with safe practices. OSAP offers extensive online resources and has developed a valuable water safety toolkit, which includes questions to help guide selection of waterline devices and chemical treatment options, and a checklist for dental unit water quality improvement. These can be found at osap.org/page/issues_DUWL.
EPA: The EPA oversees and approves disinfectants for waterlines and waterline devices. It is important to look for its approval on water disinfection products. It also sets the standard for safe drinking water. DUWS must meet drinking water standards of less than or equal to 500 CFU/mL.
Manufacturers: Each manufacturer must create an IFU for their dental unit. This IFU will include instructions for care of the waterline and a standard CFU count. Those can vary from 200 to 500 CFU/mL. It is important to read the IFU to know the count to follow. Use the unit following the cleaning and disinfection procedures in the manufacturer’s reprocessing instructions.
IFU remind the end user to only use disinfecting agents approved by the unit’s manufacturer. Some chemicals may not be compatible with your unit and could do damage to the lines, creating rough surfaces where more biofilm can form, or can be toxic to patients.
Never attach devices to a waterline that has not been cleaned or disinfected according to IFU. Test and resolve any issues beforehand. All manufacturers must provide validated instructions for reprocessing; provide patient safety information; and have test and treatment recommendations if identifying a specific product. They must notify new and past customers of updated instructions and recommendations. If your IFU does not include recommendations for waterline maintenance, call the manufacturer directly. Request their requirements in writing. Dental sales representatives’ opinions are not the same as following an IFU.
Local entities: Dental boards and health departments represent the needs of the consumer and the DHCP. They are there to help with compliance and create laws and relations to keep everyone safe. In the event of a DUWS failure that results in disease transmission, they will investigate, enforce, and aid in getting offices into compliance.
Author’s note: Next article, we will dive into part two of this series and see how a study at Cabrillo College uncovered several important keys to waterline maintenance. A special thanks to the class of 2018 and Cabrillo College for their dedication to this research project: Amanda Anfield, Stephanie Archibald, Sarah Augenstein, Maria Becerra, Jessica Carrera, Kristan Castro, Daniel Donoghue, Jordan Durrer, Adriana Garza, Esther Gonzales, Amanda Mason, Sara Nelson, Thomas Nop, Annalisa Ortiz, Sierra Partridge, Michelle Peterson, Thi Phan, Kathlyn Romero, Halley Santaella, Katia Serrano, Lorena Thomas, Michelle Van Schoick.
Updated November 29, 2022
References
1. Blake GC. The incidence and control of bacterial infection in the dental spray reservoirs. Brit Dent J. 1963:115(10):413-416.
2. Barbeau J. Waterborne biofilms and dentistry: the changing face of infection control. J Can Dent Assoc. 2000;66(10):539-541.
3. Williams JF, Johnston AM, Johnson B, Huntington MK, Mackenzie CD. Microbial contamination of dental unit waterlines: prevalence, intensity and microbiological characteristics. J Am Dent Assoc. 1993;124(10):59-65.
4. Atlas RM, Williams JF, Huntington MK. Legionella contamination of dental-unit waters. Appl Environ Microbiol. 1995;61(4):1208-1213.
5. Barbeau J, Gauthier C, Payment P. Biofilms, infectious agents, and dental unit waterlines: a review. Can J Microbiol. 1998;44(11):1019-1028.
6. Challacombe SJ, Fernandes L. Detecting Legionella pneumophila in water systems: a comparison of various dental units. J Am Dent Assoc. 1995;126(5):603-608.
7. Porteous N, Sun Y, Schoolfield J. Evaluation of 3 dental unit waterline contamination testing methods. Gen Dent. 2015;63(1):41-47.
8. Momeni SS, Tomline N, Ruby JD, Dasanayake AP. Evaluation of in-office dental unit waterline testing. Gen Dent. 2012;60(3):142-147.
9. Szymanska J. Bacterial contamination of water in dental unit reservoirs. Ann Agric Environ Med. 2007;14(1):137-140.
10. Walker JT, Bradshaw DJ, Bennett AM, Fulford MR, Martin MV, Marsh PD. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol. 2000;66(8):3363-3367.
11. Hatzenbuehler LA, Tobin-D’Angelo M, Drenzek C, et al. Pediatric dental clinic-associated outbreak of Mycobacterium abscessus infection. J Pediatric Infect Dis Soc. 2017;6(3):e116-e122. doi: 10.1093/jpids/pix065.
12. Peralta G, Tobin-D’Angelo M, Parham A, et al. Notes from the field: Mycobacterium abscessus infections among patients of a pediatric dentistry practice—Georgia, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(13):355-6. doi: 10.15585/mmwr.mm6513a5.
13. Drinking Water Contaminants – Standards and Regulations. United States Environmental Protection Agency website. https://www.epa.gov/dwstandardsregulations. Accessed November 30, 2018.
14. Wu H, Moser C, Wang HZ, Høiby N, Song, ZJ. Strategies for combating bacterial biofilm infections. Int J Oral Sci. 2015;7(1):1-7. doi:10.1038/ijos.2014.65
15. Donlan R. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881-890.
16. US Food and Drug Administration. Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm253010.pdf. Published March 17, 2015.
17. Kohn WG, Collins AS, Cleveland JL, et al.; Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003 Dec 19;52(RR-17):1-61.
18. ADA Science Institute. Oral Health Topics: Dental Unit Waterlines. American Dental Association website. https://www.ada.org/en/member-center/oral-health-topics/dental-unit-waterlines. Updated October 12, 2018.
About the Author
Noel Kelsch, MS, RDHAP
Noel Kelsch, MS, RDHAP, is a syndicated columnist, researcher, writer, speaker, and cartoonist. She is the director of Cabrillo College dental hygiene program, past president of the California Dental Hygienists ‘Association, and immediate past president of the Dental Hygiene Board of California. She owns her own dental hygiene practice that facilitates care for those dealing with homelessness and hospice care. She has received many national awards and may be reached at [email protected].